Summary
The recombining sequence (RS) of mouse and its human equivalent, the kappa deleting element (kde), are sequences found at the 3′ end of the immunoglobulin κ locus that rearrange to inactivate the κ-locus in developing B cells. RS recombination correlates with Igλ expression and plays a role in receptor editing by eliminating κ genes encoding autoantibodies. A mouse strain was generated in which the recombination signal of RS was removed in the germline, a model predicted to prevent specifically a fraction of receptor editing events. In RS mutant mice receptor editing and self-tolerance were impaired, in some cases leading to autoantibody formation. Surprisingly, RS mutant mice made fewer λ B cells, whereas λ/ κ isotype exclusion was only modestly affected. These results may provide insight into the mechanism of L-chain isotype exclusion and indicate that RS has a physiological role in λ B cell formation.
Keywords: receptor editing, recombining sequence, kappa deleting element, immune tolerance
Introduction
The quasi-random nature of the B cell antigen receptor (BCR) gene assembly from variable (V), diversity (D) and joining (J) elements by the V(D)J recombinase machinery creates several quality control problems, including the production of cells carrying useless or dangerous specificities, and cells lacking any antigen receptor at all owing to out of frame rearrangements. Positive selection mechanisms, such as assembly of the preBCR and BCR complexes, promote survival and development of cells with antigen receptors and disfavor cells carrying out of frame rearrangements (Kitamura and Rajewsky, 1992; Spanopoulou et al., 1994; Young et al., 1994; Papavasiliou et al., 1995; Papavasiliou et al., 1996; Tze et al., 2005; Verkoczy et al., 2007). Negative selection by contrast reduces the frequency of cells carrying autoreactive receptors through the mechanisms of clonal elimination or receptor editing (Goodnow et al., 1989; Nemazee and Burki, 1989; Erikson et al., 1991; Russell et al., 1991; Cyster et al., 1994).
In the process of receptor editing B lymphocytes alter their antigen receptors through secondary antibody gene rearrangements, usually involving the κ and λ light chain gene loci. The editing response often occurs in developing B cells that encounter autoantigens, resulting in the rescue of cells with reduced or abolished autoreactivity. Receptor editing is particularly facilitated by the unique structure of the Igκ locus, which promotes secondary recombinations that can replace or destroy active κ genes. Multiple sequential κ locus recombinations can occur on a single allele owing to the presence of four functional J elements which can recombine to upstream V elements (Feddersen and Van Ness, 1985; Shapiro and Weigert, 1987).
One DNA element that is predicted to have an exclusive role in receptor editing and the silencing of κ light chain genes is the Recombining Sequence (RS) (Durdik et al., 1984). RS is the mouse homologue of the immunoglobulin kappa deleting element (κde) of humans (Siminovitch et al., 1987). It lies ~25kb downstream of the Cκ exon, carries a canonical Ag receptor gene recombination signal with a two-turn spacer, and rearranges by V(D)J recombination to sites upstream, including to germline Vκ elements and to two sites in the Jκ-Cκ intron (IRS1 and IRS2) (Durdik et al., 1984; Moore et al., 1985; Siminovitch et al., 1985; Siminovitch et al., 1987; Klobeck and Zachau, 1986; Shimizu et al., 1991; Selsing and Daitch, 1995). RS recombination results in the physical deletion of Cκ and the silencing of the κ locus. This natural “κ knockout” process frequently occurs in the development of mammalian B cells. Almost all mouse and human Igλ B cells (75–95%), and a significant subset of Igκ B cells (10–15%), carry RS recombinations (Durdik et al., 1984; Moore et al., 1985; Siminovitch et al., 1985; Nadel et al., 1990; Zou et al., 1993; Dunda and Corcos, 1997; Brauninger et al., 2001). In actively rearranging transformed B cells, RS/kde and λ recombination are temporally and developmentally correlated, but with RS recombination preceding λ recombination (Persiani et al., 1987; Muller and Reth, 1988; Klein et al., 2005).
Because of the strong link between RS rearrangements and a cell’s Ig the presence of expression, Selsing and colleagues proposed that RS recombination might be required to trigger λ locus recombination (Durdik et al., 1984; Moore et al., 1985; Siminovitch et al., 1987; Persiani et al., 1987; Daitch et al., 1992; Selsing and Daitch, 1995). RS rearrangement might “activate” RS (or a locus downstream of RS) that would in turn promote λ recombination (Persiani et al., 1987). Alternatively, RS rearrangement might eliminate a putative cis-acting suppressor of λ recombination lying between RS and its recombination partner sites. Such a suppressor might be associated with transcriptional enhancers themselves as the RS element is downstream of both intronic and 3′ κ enhancers (Muller et al., 1990). However, little evidence has been obtained to support these models: RS does not recombine in a fixed frame relative to its recombination partners Vκ, IRS1 and IRS2 (Daitch et al., 1992). Moreover, and notwithstanding that RS transcription occurs in potentially recombining cells, the RS element does not appear to encode any protein, and the major homology between mouse and human RS elements is in their eptamer/nonamer recognition sites (Siminovitch et al., 1987). Immediately downstream of RS lies a housekeeping gene that is unlikely to play any B cell specific role (Apel et al., 1995). Furthermore, mouse strains carrying different targeted knockouts in the Igκ locus appeared to have robust λ-locus recombination and B cell generation despite an impaired ability to recombine RS (Chen et al., 1993; Takeda et al., 1993; Zou et al., 1993).
However, it is not excluded that RS recombination, through deletion of enhancer elements adjacent to Cκ, might promote λ-locus recombination by eliminating potentially competing DNA elements (Daitch et al., 1992; Inlay et al., 2002).
We proposed an alternative hypothesis for RS function, namely that it plays a role in receptor editing (Tiegs et al., 1993; Retter and Nemazee, 1998), a possibility also discussed by Selsing and Daitch (Selsing and Daitch, 1995). RS recombination is elevated in artificial models in which B cells are arranged to be initially autoreactive (Chen et al., 1997; Pelanda et al., 1997; Aït-Azzouzene et al., 2005). RS recombinations often inactivate previously in-frame, functional Igκ loci even in normal mice (Retter and Nemazee, 1998) and humans (Brauninger et al., 2001). In our study, 47% of such Vκ-Jκ remnant loci were in-frame, arguing that RS recombination was actively promoted by BCR signaling, presumably because of autoreactivity (Retter and Nemazee, 1998). We also found that RS recombinations usually occur when other options on the κ-locus run out, as 80% of the κ loci in sIgλ+ cells were inactivated by RS after rearranging the last J element, Jκ5 (Retter and Nemazee, 1998). We proposed that the RS locus is specialized to destroy autoreactive κ genes, and to reduce the frequency of cells with two different L-chains, allowing receptor editing to be compatible with lymphocyte monospecificity (“allelic/haplotype exclusion”). Increased RS recombination and λ B cell production associated with receptor editing were also described in autoantibody transgenic models (Tiegs et al., 1993; Pelanda et al., 1997; Li et al., 2004). More recently, we investigated mice expressing a synthetic superantigen gene that drives ubiquitous expression of a cell surface protein reactive with immunoglobulin κ. Bone marrow B cells in mice carrying the so-called κ-macroself transgene undergo massive κ-to-λ editing characterized by increased RS recombination and increased λ B cell production (Aït-Azzouzene et al., 2005).
To investigate the role of RS in λ B cell production and receptor editing, we generated a mouse mutant lacking the RS element’s heptamer/nonamer recombination signal. These mice manifest defects both in the ability to undergo receptor editing in response to autoantigen and in the generation of λ B cells. These studies also reveal a role for receptor editing in preventing autoantibody formation.
Results
Generating the RS−/− mouse
Knockout mice were generated using the scheme outlined in Supplementary Fig 1 in which the recombination signal of the RS element was removed and replaced with a neomycin resistance gene flanked by loxP sites. After cre-mediated deletion, the modified locus was verified to have the predicted sequence in which 139 bp encompassing the recombination signal of RS was substituted with a 199 bp stretch carrying a single loxP site and flanking vector sequences.
Assessing the success of the knockout
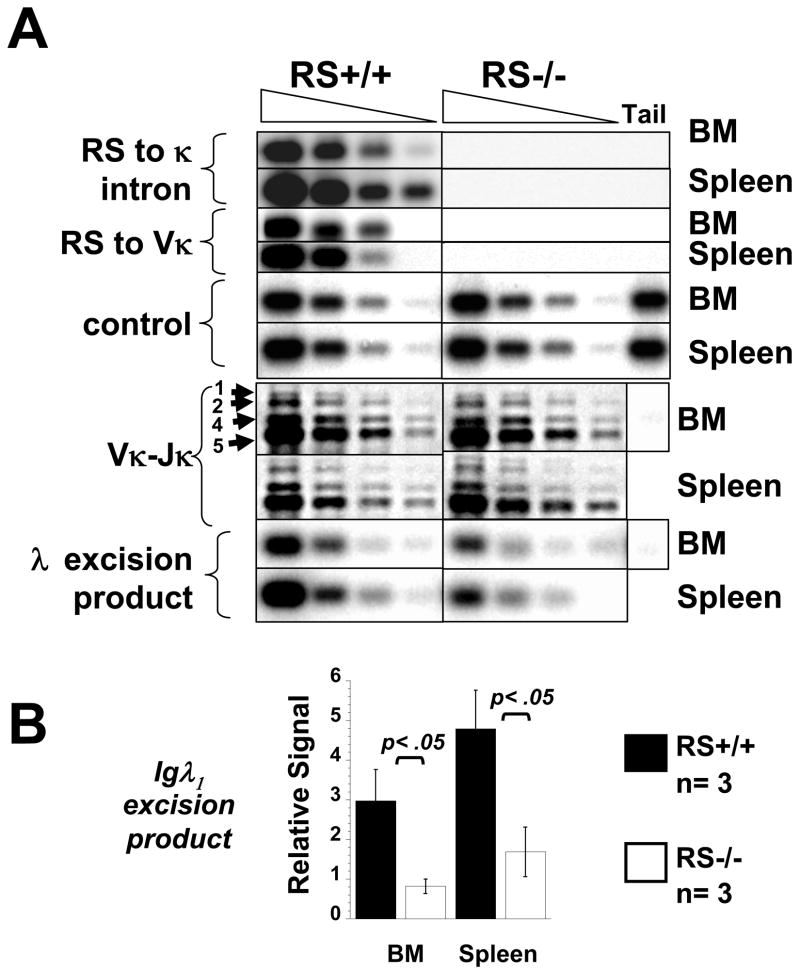
To evaluate functional effects of the RS mutation, we bred homozygous mutant (RS−/ −) mice and measured RS recombination to known recombination signal sites in Vκ and the Jκ-Cκ intron using PCR assays of spleen and bone marrow (BM) cells. As shown in Fig 1, the RS knockout had the intended effect of blocking RS type recombination to Vκ (Vκ-RS) or to the major κ intronic site (RS to κ intron) (Fig 1A, top panels). By contrast, Vκ to Jκ recombination appeared to be normal (Fig 1A). We conclude that the germline RS mutation prevented normal RS recombination and had little effect on other recombinations at the κ-locus.
Figure 1.
Evaluation of RS recombination in spleen and BM B220+ cells of RS−/− and littermate RS+/+ mice. Four-fold serial dilutions of the indicated DNA samples were subjected to PCR reactions to detect the indicated DNA rearrangements. PCR products were electrophoresed on agarose gels, blotted, and abundance quantitated by southern blot using specific probes. A) Upper panels, PCR detection of RS recombination to JCκ intronic sites (RS to κ intron) and Vκ sites (RS to Vκ). Below is shown recombinations between Vκ and Jκ (Vκ-Jκ) and excision products of Vλ1 to Jλ1 recombination (λ excision product). Similar results were obtained in at least two additional independent experiments. B) Quantitation of λ excision product levels in a sample of 3 mice/group.
As we discovered that λ B cell frequencies were reduced in RS−/ − mice (see below) we also measured λ excision product DNA levels in spleen and BM as an indicator of λ B cell production. PCR quantitation of the generation of Igλ1 recombination excision circles in the BM B220+/IgD− cells revealed a 60–70% reduction in RS−/ − mice compared to wild type (Fig 1A lower panel, Fig 1B). Because DNA excision circles are not believed to replicate and are usually the result of non-functional recombination, these results indicate a reduced rate of Igλ recombination in developing RS−/ − B cells. Hence RS mutation was correlated with a significant, but incomplete, suppression of Vλ-Jλ recombination.
Reduction in the frequency of λ B cells in RS−/− lymphocytes
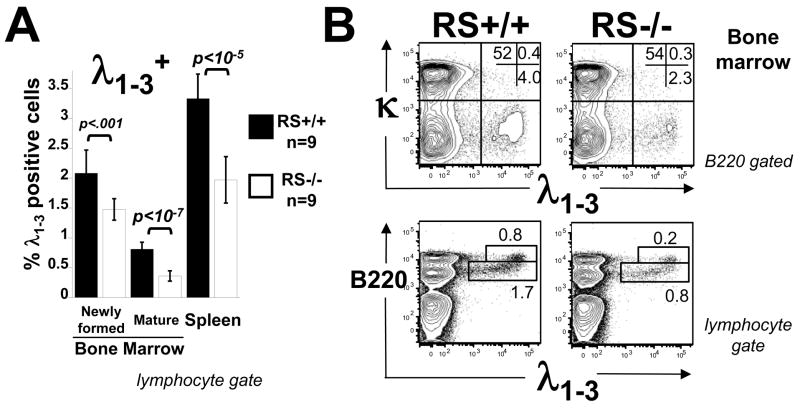
Flow cytometry analysis carried out on lymphoid tissues in a comparative sample of 9 mice per group revealed that the frequency of λ1–3 B cells in the spleens of RS−/ − mice was reduced by ~42% compared to wild type (Fig 2A, right), which was mirrored in the decrease in absolute λ1–3B cell number (Supplementary Table 1). Similar significant reductions of λ1–3B cells were seen in the BM, including among newly formed and recirculating cells (Fig 2A,B), and in all other tissues examined, including lymph nodes, peritoneal cavity and blood (Supplementary Fig 2). κ B cell numbers were normal or slightly reduced in analyses involving a total of 18 mice/group (Supplementary Table 1). These results support the conclusion that RS mutation does not completely block λ B cell generation, but lowers significantly λ B cell output from the BM and their steady state numbers in the spleen.
Figure 2.
Evaluation of reduced λ B cell frequencies in RS mutant mice. B cells from the BM and spleens of RS−/− and RS+/+ mice were stained with antibodies to CD45R (B220), Igκ, and Igλ1–3and analyzed by multicolor flow cytometry. A) Frequencies of cells carrying Igλ 1–3 in the spleen and in newly formed (B220intermediate) and mature (B220high) BM B cells. B) Upper plot shows costaining for κ and λ in BMs of the indicated mice. Lower panels, gating used in A to distinguish newly formed from recirculating sIg+ B cells in BM.
Hybridoma analysis
To further probe the effect of RS mutation, a panel of B cell hybridomas was generated and analyzed for antibody L chain type and RS gene status (Table 1). Among 244 hybridomas generated from 2 wild type mice, 22 (9%) secreted Igλ, whereas among hybridomas generated in 5 fusions of RS mutant mice only 2.8% were λ+ (17 of 603 hybridomas). Intracellular staining and flow cytometry analysis of λ cells failed to detect cells coexpressing both κ and λ (data not shown). We conclude that, as sampled by hybridoma analysis, the frequency of λ expressing B cells is reduced in RS mutant mice and that cells expressing both κ and λ simultaneously are remarkably rare, regardless of RS mutation.
Table 1.
Analysis of B cell hybridomas from RS−/− and RS+/+ mice for λ 1–3 secretion, κ/λ 1–3 protein coproduction, and RS recombination.
| a Fusion number | Mouse genotype | λ1–3(%) | λ+/ #screened | λ+/κ+ | λ+/κ | Total λ+ frequency | RS status of λ+ hybrids #rearranged / #tested | bRS recombination in λ+ hybridomas |
|---|---|---|---|---|---|---|---|---|
| 1 | RS+/+ | 7.4 | 2/27 | 0 | 2 | 9 % (22/244) | 1/1 | 100% (8/8) |
| 2 | 9 | 20/217 | 0 | 20 | 7/7 | |||
| 3 | RS−/− | 2.2 | 4/180 | 0 | 4 | 2.8% (17/603) | 0/4 | 0% (0/9) |
| 4 | 5 | 4/80 | 0 | 4 | 0/3 | |||
| 5 | 5 | 3/61 | 0 | 3 | 0/2 | |||
| 6 | 1 | 1/92 | 0 | 1 | ||||
| 7 | 2.6 | 5/190 | 0 | 5 |
Each line represents a single fusion experiment using spleen cells from a different individual mouse. Hybridoma clones were screened for κ and λ1–3immunoglobulin production as indicated in Experimental Procedures.
RS rearrangement status was assessed by PCR assay and genomic southern blotting. Hybridomas were scored positive if at least one RS rearrangement was detected.
As RS is usually recombined in λ-producing B cells, we tested λ-expressing hybridomas from mutant and wildtype mice for RS rearrangements by PCR as in Fig 1A, and by southern blotting using different restriction enzymes in conjunction with an RS probe. Nine of 9 tested RS−/ − hybridomas lacked detectable RS rearrangements, whereas RS rearrangements were readily detected in wildtype λ producing B cells (Table 1). These results supported the PCR assays of primary B cells (Fig 1A) in indicating that the mutant RS element does not detectably rearrange, even in B cells that eventually express λ chain. We conclude that although RS recombination does facilitate λ B cell development, significant λ B cell development is possible in the apparent absence of RS element rearrangement.
Analysis of VκJκ5 joins in λ B cells
To test if the reduction in λ cells in RS−/ − mice was the result of counterselection of B cells with functional κ loci, we next cloned and sequenced VκJκ5 joins from sorted λ+ B cells. (In wild type mice, RS recombination often silences functional κ loci, usually leaving behind remnant Vκ joins involving the last Jκ element, Jκ5 (Retter and Nemazee, 1998).) We indeed found evidence of counterselection because only 10% (6/60) of VκJκ5 joins from RS−/ − B cells were potentially productive compared to 34% (18/53) of wild type joins. Such putative counterselection could occur before or after any actual λ recombination (see Discussion). In any case, this finding is consistent with the notion that λ B cells are often derived from κ cells carrying forbidden receptors that are silenced by RS-mediated editing.
Serum antibody analysis
RS−/ − mice had comparable serum immunoglobulin levels to wild type, except that IgM, λ levels were reduced about 44% (10 μg/ml vs 17.9 μg/ml, p=.0016, n=9). To see if RS−/ − mice had elevated spontaneous autoantibody levels, we measured IgM and IgG anti-dsDNA levels in sera of seven month old mice by ELISA. not statistically significant, there was a trend to higher level binding in the RS−/ − group (Fig 3A). C57BL/6 mice normally do not develop anti-DNA antibodies before 12 months of age (Morel et al., 1997). These tests for autoantibody were repeated using serum from a cohort of RS knockout mice carrying a B-lineage restricted Bcl2 transgene (Strasser et al., 1990) and compared to results from RS+/+;Bcl2 Tg mice. In the context of enforced Bcl2 expression in B cells the RS mutation significantly augmented anti-dsDNA levels over those present in RS+/+;Bcl2 mice (Fig 3B).
Figure 3.
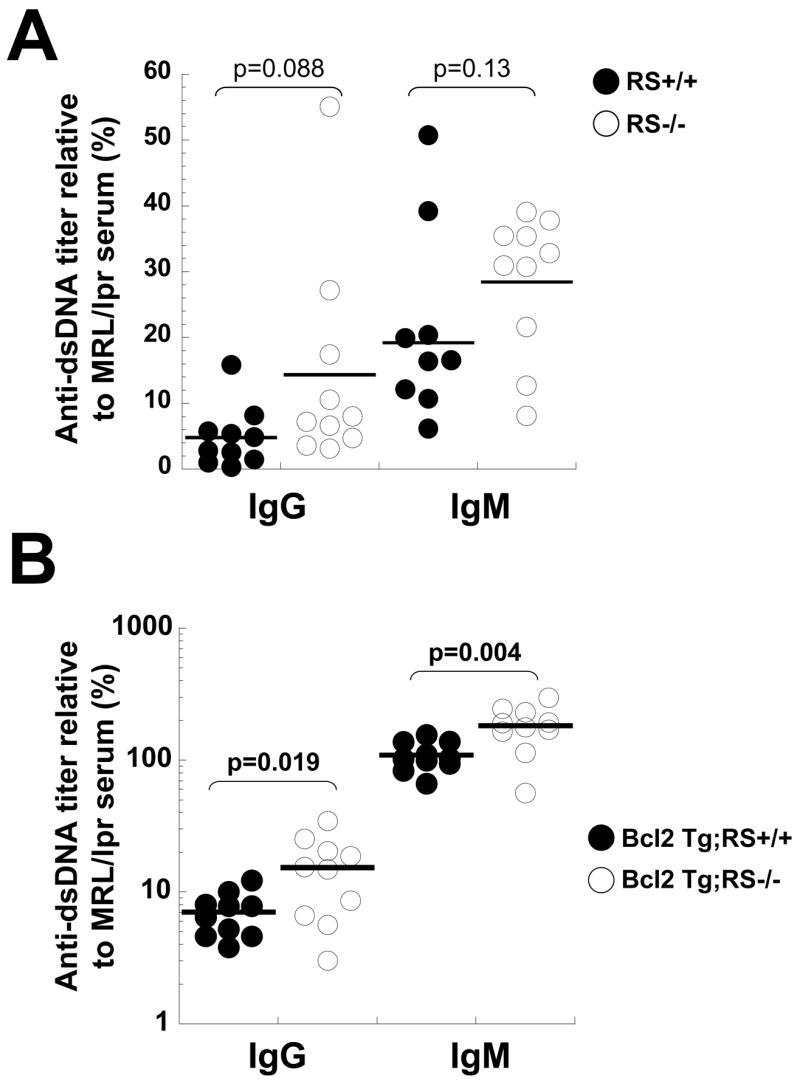
Analysis of anti-dsDNA antibody in sera of the RS mutant and wild type mice. A) Anti-dsDNA titers in seven month old RS+/+ and RS−/− mice. B) Anti-dsDNA titers in 5 month old Bcl2 Tg:RS+/+ and Bcl2 Tg;RS−/− mice. Each dot represents the value obtained from a different individual mouse.
Effects of RS mutation on receptor editing
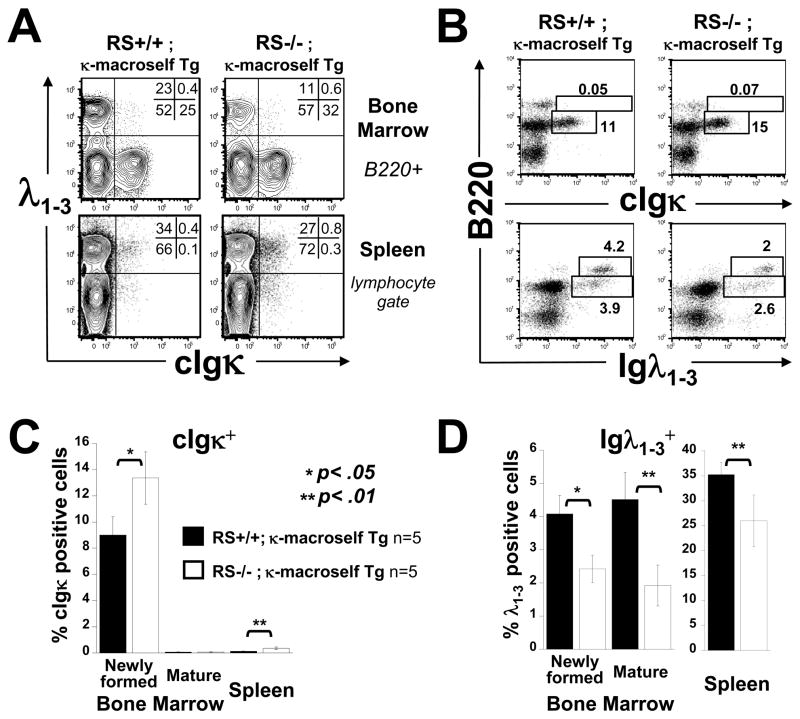
To measure the effect of RS mutation on central tolerance, we bred RS−/ − mice to transgenic mice expressing ubiquitously a membrane-tethered Igκ superantigen (κ-macroself Tg mice)(Aït-Azzouzene et al., 2005) and assessed B cells numbers and phenotype by flow cytometry. In this context all Igκ B cells are self reactive. We previously showed that developing B cells in κ-macroself Tg mice undergo increased RS recombination and massive κ-to-λ editing. These mice essentially lack peripheral κ cells, but have a modest increase in the steady state frequency of BM κ cells. Most importantly, κ-macroself Tg mice have a 3-4-fold increase in the BM production of λ cells and a 7-fold increase in λ cells in the spleen (Aït-Azzouzene et al., 2005). RS mutant mice bred to the κ-macroself Tg background were found to have fewer λ cells in the spleen compared RS-sufficient κ-macroself Tg controls (ca. 25% vs 35%, Fig 4 A,D). The frequency of newly formed BM λ cells compared to RS-sufficient controls was similarly reduced in RS−/ −;κ-macroself Tg mice (Fig 4 B,D). It appears that λ B cell development in the context of a Igκ-reactive superantigen is partly impaired in RS−/ − mice.
Figure 4.
Analysis of IgL-chain expression of RS mutant B cells developing in the presence of a κ superantigen. A-D) Spleen or BM cells of the indicated mice were simultaneously analyzed by multicolor flow cytometry for surface expression of Igλ and cytoplasmic expression of κ (cIgκ). Analyzed cells were gated on lymphocytes unless otherwise indicated.
As expected, the κ-macroself antigen induced a loss of κ cells in the spleen in both RS-sufficient and deficient mice. But in RS mutants there was a slight increase in the numbers of κ cells escaping to the periphery (Fig 4A,C). Moreover, in the BM of RS−/ −;κ-macroself Tg mice the frequency of immature B220intermediate/κ cells was significantly increased (Fig 4B,C). These additional, functionally autoreactive κ cells presumably represent those cells that were unable to silence κ loci by RS recombination. We expected to also see a substantial population of κ/ λ double positive cells in the BMs of RS−/ −;κ-macroself Tg mice, however, the κ/ λ population was relatively small. We conclude that in the context of central negative selection of κ cells the RS mutation leads to a significant reduction in λ cells, and a slight increase in κ cells appearing in the peripheral immune system.
Complementary tolerance mechanisms revealed in RS−/− B cells with enforced expression of Bcl2
In order to test the possibility that the reduction in λ B cell production in RS−/ − mice was the result of the rapid death of κ cells carrying autoreactive receptors, we bred RS knockout mice to the B-lineage restricted Bcl2 transgenic mouse (Strasser et al., 1990) and used their BM cells to reconstitute lethally irradiated mice carrying the κ-macroself Ag. The choice of a BM transfer approach to challenge B cells with the superantigen (as opposed to introducing the superantigen by breeding) was one of convenience and also ensured that the superantigen was not expressed on B cells themselves. We reasoned that the κ-macroself Ag would induce κ-to-λ editing and that enforced Bcl2 expression would potentially rescue survival of κ/ λ double positive cells that might be generated in the absence of RS. The following differences were apparent in the recipients of RS−/ −;Bcl2Tg compared to RS+/+;Bcl2Tg cells. First, the striking loss of BM κ+ cells in the presence of κ-macroself Ag failed to occur in the recipients of RS−/ −;Bcl2Tg cells, indicating that much of the κ B cell loss in RS+/+;Bcl2Tg mice was the result of RS-mediated editing, rather than mere BCR downregulation (Fig 5A a,b, lower right quadrants). Second, in RS−/ − mice fewer λ positive cells were generated in the BM (Fig 5B, Fig 5A, compare a,b, upper left quadrants) and spleen (Fig 5B, Fig 5A plots c,d). Third, the expected increase in κ/ λ double positive cells failed to occur. Finally, κ cells, which were not seen in the spleens of κ-macroself Tg recipients of RS+/+;Bcl2Tg BM, were present in large numbers in spleens of mice that received RS−/ −;Bcl2Tg BM (Fig 5Ac,d). Many of these κ splenocytes expressed markers of maturation, including CD21 and CD23, but they also expressed CD93 (Fig S3). These findings indicate that RS mutants have a defect in self-tolerance but a modest impairment in κ/ λ isotype exclusion.
Figure 5.
Analysis of the effect of RS mutation on B cell tolerance in apoptosis-resistant cells. BM chimeras were generated using donor BM from RS-sufficient or RS-mutant mice that also carried a Bcl2 transgene enforcing B lineage restricted expression. Irradiated recipient mice carried a ubiquitously expressed κ-macroself transgene (Aït-Azzouzene et al., 2005) to promote negative selection of κ+ cells. Radiation chimeras were analyzed at 10 weeks post reconstitution. A) Analysis of λ and κ cell frequencies in BM and spleens of the indicated mice. (Note that κ staining in this figure involves surface staining, rather than the cytoplasmic staining of permeabilized cells as shown in Fig. 4A.) B,C) Summary analysis of the κ and λ frequencies of total splenic cells, and newly formed (B220intermediate) and recirculating B cells (B220high) from the indicated chimeras. Note increased κ frequency and reduced λ frequency among BM and spleen cells of κ-macroself recipients of Bcl2 Tg;RS−/− bone marrow. D) Igκ and Igλ serum immunoglobulin levels measured in the indicated chimeric mice.
To determine if the autoreactive B cells rescued in RS−/ −;Bcl2 Tg→ κ-macroself Tg mice were functional, we assessed in these mice serum κ immunoglobulin levels. As shown in Figure 5D, high serum levels of Igκ were found in IgM and IgG isotype fractions of animals receiving RS−/ −, but not RS+/+ cells. We conclude that the Bcl2 transgene can indeed promote survival and maturation of autoreactive B cells and that a defect in RS editing (the RS mutation) combined with a pure defect in cell survival (Bcl2Tg) can lead to the escape of autoreactive B cells to the periphery. Moreover, because in recipients of RS+/+;Bcl2Tg BM κ+ cells were rare in the spleen, these results indicate that RS-mediated editing efficiently “eliminated” autoreactive B cells despite their apoptotic defect.
Discussion
The RS/κde element has been suggested to play an evolutionarily conserved role in three important and possibly interrelated physiological processes: the regulation of Igλ gene expression, κ/ λ L-chain isotype exclusion, and receptor editing (Durdik et al., 1984; Siminovitch et al., 1985; Siminovitch et al., 1987; Muller and Reth, 1988; Muller et al., 1990). These effects were presumed to involve directly or indirectly RS recombination itself. However, until now few direct experiments have been carried out to specifically assess RS function. A specific RNA transcript is associated with the RS locus, which may play a role in recombinase accessibility, but no RS protein coding function is likely (Daitch et al., 1992). We show here that a targeted mutation of the RS recombination signal blocked RS recombination, allowing an assessment of its putative functions. RS mutant mice had defective λ B cell production, but surprisingly normal κ/ λ isotype exclusion. Most importantly, we have generated a mutant with a targeted defect in receptor editing and have demonstrated that this defect can abrogate tolerance, particularly in conjunction with an apoptotic defect contributed by enforced Bcl2 expression.
To our knowledge this is the first demonstration of a specific defect in receptor editing that promotes autoantibody formation. In other studies, mice with defective editing were engineered by rendering autoantibody transgenic mice RAG gene deficient, however, these mice manifested increased B cell deletion, rather than autoantibody formation (Spanopoulou et al., 1994; Xu et al., 1998; Halverson et al., 2004). Similarly, conventional autoantibody transgenic mice have been bred to a background expressing a B cell restricted Bcl2 transgene (Hartley et al., 1993; Lang et al., 1997). In that context, central tolerance and editing appeared to be intact, and overt autoantibody formation was minimal. However, in mice in which B cells encountered antigen solely in the periphery, presumably at a developmental stage at which they were no longer competent to undergo receptor editing, self-tolerance was broken by Bcl2 overexpression (Lang et al., 1997). In the present study and earlier work we found that Bcl2 overexpression alone did not hinder receptor editing, and central tolerance proceeded efficiently (Lang et al., 1997; Aït-Azzouzene et al., 2005). However, we show here that Bcl2 overexpression combined with the RS mutation led to frank autoimmunity. Our findings reinforce the suggestion that central tolerance by receptor editing is normally complemented by developmental arrest and apoptosis in cells that fail to edit in an appropriate time frame (Spanopoulou et al., 1994; Lang et al., 1997; Xu et al., 1998; Halverson et al., 2004).
Compared to mice, humans have a much higher proportion of B cells that express Igλ L-chain (40% vs 6%) and that have rearranged the RS/kde element (50% vs 20%) (Durdik et al., 1984; Siminovitch et al., 1985; Dunda and Corcos, 1997; Brauninger et al., 2001). It therefore seems likely that the kde element plays an even more important role in humans than does the RS element of mice.
Lack of a functional recombination signal adjacent to RS suppressed significantly both λ B cell production and κ-to-λ receptor editing, but this mutation affected κ/ λ isotype exclusion only to a modest degree. It has been noted before that B cells expressing both κ and λ are rare, but detectable (Zou et al., 1993; Pauza et al., 1993; Gollahon et al., 1988; Giachino et al., 1995; Diaw et al., 2000). However, it is interesting in this regard that, as in our study, few hybridomas could be isolated from normal mice that coexpress κ and λ (Gollahon et al., 1988). The frequency of B cells with κ-chain allelic inclusion has been estimated to be from 1.5% (Casellas et al., 2001) to 10% (Casellas et al., 2007). The latter study concluded that most cells that scored as included expressed only one chain on the cell surface. We found that 10% (6/60) of λ splenic B cells in RS−/ − mice carried an in-frame κ rearrangement. The significance of the remaining in-frame κ genes is unclear. Some may represent contamination of κ cells, others rearranged κ genes rendered non functional by defects outside of the region sequenced or cells carrying κ chains that are poorly expressed or unable to pair with the cell’s H-chain. In our hybridoma analysis we failed to identify any κ/ λ protein double expressing hybrids from RS−/ − or wild type mice. In any case, our results indicating no striking increase in κ/ λ inclusion in RS−/ − mice appear to demand the counter-intuitive conclusion that the RS element plays a minor role in enforcing κ/ λ isotype exclusion, despite its critical role in promoting κ-to-λ editing.
It is of interest to contrast our conclusions with earlier studies involving targeted mutations of the κ locus. These include targetings that introduced neomycin genes within the J-C region of the κ locus (Takeda et al., 1993; Chen et al., 1993; Zou et al., 1993) and others that involved deletions of transcriptional regulatory elements with minimal introduction of heterologous sequence (Xu et al., 1996; Inlay et al., 2002). Many of these mutations blocked both functional κ expression and RS recombination owing to defects in cis-acting transcriptional regulators that affect recombinational targeting. These combined defects in κ expression and RS recombination were correlated with greatly increased, rather than decreased, λ locus recombination and expression, and appeared to exclude a required role for RS recombination in Igλ recombination and expression. Our present study permitted functional κ expression in the absence of RS recombination, revealing a specific, but partial defect in Igλ recombination and λ B cell production as a consequence of defective RS recombination.
How does RS mutation suppress λ gene expression? The receptor editing view of RS function is that its purpose is to destroy κ loci encoding autoreactive receptors, allowing progression to λ (Tiegs et al., 1993; Chen et al., 1997; Pelanda et al., 1997; Retter and Nemazee, 1998). One might suppose that without RS, autoreactive cells would be eliminated by clonal deletion or, failing deletion, they would become allelically included. Several studies indicate that failed editing of developing autoreactive B cells leads to deletion (Spanopoulou et al., 1994; Xu et al., 1998; Halverson et al., 2004). However, our data indicate that the dearth of λ cells in RS mutants cannot be attributed simply to the death of autoreactive κ B cells that fail to edit using RS. Even under artificial conditions in which apoptotic deletion of demonstrably autoreactive κ B cells was hindered by Bcl2 overexpression, λ B cell numbers were reduced and κ/ λ double positive cells were rare. Our results appear to rule out premature clonal deletion as an explanation for how λ B cell production is reduced in RS−/ − mice. Rather, the results support the previously abandoned suggestion that RS recombination is required to promote Igλ rearrangement (Durdik et al., 1984).
If RS recombination was needed to activate λ recombination directly, for example, through the activation of a trans-acting gene product encoded in the κ locus, mutants impaired in the ability to recombine RS should lack λ cells. However, several published experiments involving targeting of other elements in the κ locus have shown that λ B cells can be generated in the absence of RS recombination, often at enhanced efficiency (Chen et al., 1993; Takeda et al., 1993; Zou et al., 1993; Aït-Azzouzene et al., 2005). Moreover, analysis of germline λ transcription in BM B cells of RS−/ −;Bcl2 Tg→ κ-macroself Tg chimeras suggests that RS mutation does not reduce accessibility of the λ locus (Fig S4).
An alternative hypothesis to explain the linkage between RS recombination and λ locus recombination invokes a putative enhancer competition for recombinational targeting. As RS recombination deletes the Cκ locus, it is predicted to change the context of the major cis acting elements adjacent to Cκ. In the absence of these competing cis acting elements, recombinational targeting to the λ loci might be more efficient. However, we do not favor this hypothesis for the following reasons. First, because excision circles formed by RS recombination are generated in non dividing cells, these often large episomes are not typically lost, so would in theory be available to provide competition in binding to any recombinational targeting machinery. Second, κ-macroself Tg mice on a RS-sufficient background generate λ B cells just as efficiently as do JCκ deleted mice (Aït-Azzouzene et al., 2005), though JCκ deleted mice lack intronic enhancer elements as well as any κ locus or RS recombination (Chen et al., 1993). Third, in Cκ exon targeted mice, in which Vκ-to-Jκ recombination but not RS recombination occurred, λ B cell production was indistinguishable from that of mice unable to recombine any κ loci (Zou et al., 1993; Chen et al., 1993). These considerations argue against a simple model in which competition from recombinationally active κ loci suppresses λ recombination.
Our findings suggest that the defect in λ recombination seen in RS mutant mice occurred selectively in those B cells that expressed functional κ-chain. Analysis of BM B cells using intracellular staining for Igκ indicated that immature B cells expressing λ largely lacked any detectable κ expression and vice versa. Moreover, the extent of reduction of λ B cell production in RS mutant mice (~42%) was similar to the proportion of cells predicted to normally extinguish in-frame κ genes by RS recombination (Retter and Nemazee, 1998) and few VκJκ5 junctions cloned from sorted λ+ B cells of RS−/ − mice had in-frame κ rearrangements. In contrast to κ knockout mice, in RS−/ − mice λ generation is significantly, but partly suppressed, both in the presence or absence of κ-macroself Ag. Moreover, the reduced output of λ cells in RS−/ − mice is reflected in the amount of λ recombination excision circles. That almost all λ B cells that do develop in RS mutant mice lack κ expression appears to result from specific inhibition of Igλ recombination in sIgκ+ cells, rather than counterselection of κ/ λ double positive cells after their formation.
An appealing alternative hypothesis to explain the dearth of λ B cell production in RS mutant mice is that induction of λ recombination in cells undergoing receptor editing occurs most efficiently when κ protein expression has been silenced. According to this view, the reduced λ B cell production in RS mutant mice arises from their inability to silence κ protein expression in editing cells. This hypothesis is supported by the findings that relatively few λ/κ double expressing cells are generated in the BMs of κ-macroself Ag expressing mice in which all κ cells are autoreactive and induced to carry out receptor editing, even when survival was artificially enhanced by introduction of the Bcl2 transgene. It appears that λ gene activation usually occurs selectively in cells lacking sIg, which occurs after RS mediated editing prevents κ expression. The relative inability of cells to undergo λ recombination when unable to silence expression of an autoreactive κ gene could occur if (a) immature autoreactive cells express lower recombinase levels than do sIg negative (preB) cells, (b) recombinase levels are limiting for editing and (c) λ gene recombination requires higher levels of recombinase than does κ recombination. There is evidence to support all of these possibilities. In early analyses of receptor editing in autoantibody transgenic mice, where virtually all developing B cells carried an autoreactive BCR, BM RAG mRNA levels, though high in the presence of autoantigen, were lower than in non transgenic controls dominated by small, sIg- preB cells (Tiegs et al., 1993; Lang et al., 1996). Moreover, RAG expression in BM B cells of RS−/ −;Bcl2 Tg→ κ-macroself Tg chimeras is reduced compared to RS+/+ sufficient controls (Fig S4). Furthermore, we have shown in two contexts that RAG1 heterozygous deficiency suppresses markedly receptor editing in vivo (Verkoczy et al., 2005; Aït-Azzouzene et al., 2005), indicating that RAG expression is limiting for editing. Finally, λ genes are known to be much less efficiently recombined than κ genes because their recombination signals contain more non-consensus substitutions (Ramsden and Wu, 1991). Although the recombination signal of the RS element diverges from consensus to a similar extent as λ elements, RS mainly joins with Vκ elements, which carry near consensus recombination signals (Table 2). RS recombination usually correlates with λ recombination, and proceeds after initiation of κ rearrangements, however, exceptions have been identified both in normal and genetically modified individuals (Berg et al., 1990; Nadel et al., 1990; Chen et al., 1993; Zou et al., 1993; Dunda and Corcos, 1997), indicating that λ locus recombination can sometimes precede κ recombination. These exceptions might arise because λ genes assemble best in developing B cells that by chance or mutation fail to generate κ protein, regardless of their RS recombination status. We would therefore suggest that in autoreactive B cells undergoing receptor editing (and that are therefore sIg+), RAG levels are limiting and are sufficient to drive κ and RS recombination, but generally insufficient to drive λ recombination, which we propose occurs preferentially after destructive editing first renders the cell sIg negative.
Table 2.
Analysis of recombination signal sequences of mouse antibody L chain loci relative to usage.
| 9-mer | 7-mer | non-consensus substitutions | approximate usage | |
|---|---|---|---|---|
| Consensus | GGTTTTTGT | CACTGTG | ||
| Jk1 | --------- | ------- | 0 | 33% |
| Jk2 | a-------- | ------- | 1 | 25% |
| Jk4 | --------- | ------- | 0 | 13% |
| Jk5 | --------- | ------- | 0 | 28% |
| Vk | --------- | ------- | 0–2 (Avg 0.8) | 99% |
| RS | a----c--c | ------- | 3 | 20% |
| Vlambda1 | t---c---- | --t---- | 3 | 3% |
| Vlambda2 | t---c---- | --t---- | 3 | 2% |
| VlambdaX | a----c--- | t------ | 3 | 1% |
| Jlambda1 | -t-------c | ---a--- | 3 | 3% |
| Jlambda2 | ------g-g | --t---- | 3 | 3% |
| Jlambda3 | -----ag-g | ------- | 3 | <1% |
Adapted from (Ramsden and Wu, 1991). Shown are the nonamer/heptamer elements of the signals and their deviations from the consensus at the top of the figure. The number of positions deviating from consensus were summed in the central column. The approximate usage of gene segments in total B cells of wild type mice is shown at right. Data were derived from the following references (Ramsden and Wu, 1991; Wood and Coleclough, 1984; Durdik et al., 1984; Eisen and Reilly, 1985; Nadel et al., 1990; Shimizu et al., 1991; Luning Prak et al., 1994; Dunda and Corcos, 1997).
Experimental Procedures
Generation of RS knockout construct and production of knockout mice
Please see Supplementary data and experimental procedures.
Mice
All mice were bred and maintained in the TSRI Animal Resources facility according to The Scripps Research Institute Institutional Animal Care and Use guidelines. C57BL/6J (B6) and B6.CD45.1 mice were from Jackson Laboratories. EmuBcl-2-22 transgenic (Bcl2Tg) mice (Strasser et al., 1991), were provided by Drs. Strasser and Harris (WEHI, Melbourne, Australia). κ-macroself transgenic (line 2) was described (Aït-Azzouzene et al., 2005). RS−/ − mice analyzed had been backcrossed ten times to C57BL/6J mice (B6) and were compared to B6 controls. In some experiments involving mice carrying κ macroself and Bcl2 transgenes had been backcrossed 6 times to B6 or B6.CD45.1. Bcl2 Tg mice were initially on a mixed B10D2/B6 background.
Flow cytometry analysis, serum antibody analysis and bone marrow chimeras
Flow cytometry, serum antibody analyses and radiation bone marrow chimera generation were essentially as described (Aït-Azzouzene et al., 2005). Assay for dsDNA autoantibodies was carried out as follows. 10 μg/ml dsDNA from salmon sperm was coated to Nunc Maxisorb 96 well plates in 0.5X Reacti-Bind DNA coating solution (Pierce). After overnight coating, wells were blocked for 1 hr in Tris buffered saline containing 5% non-fat dry milk powder. Mouse sera diluted in blocking solution supplemented with 1% BSA were applied and incubated for 90 min at 37° C. After extensive washing, bound antibodies were detected with 1:2000 diluted horseradish peroxidase-conjugated goat anti-mouse IgM or goat anti-mouse IgG (Jackson Immunoresearch) and developed with 1-Step-Ultra TMB colorimetric substrate (Pierce). OD450nm was measured using a Versamax plate reader (Molecular Devices). Anti-dsDNA antibody concentration was normalized to a high titer control serum kindly provided by Dr. D. Kono. Flow cytometry data collection was done with an LSRII flow cytometer (Becton Dickenson) and analyzed using FlowJo software. For intracellular staining, surface stained cells were fixed and permeabilized using a kit (Cytofix/Cytoperm; BD Biosciences) and stained according to the manufacturer’s instructions. For BM transplantation, recipient mice carried the CD45.1 marker and in some cases carried the κ-macroself Tg. All BM donors were of the CD45.2 allotype. Ten weeks post reconstitution recipients were sacrificed and their lymphoid tissues analyzed. Only chimeras in which ≥98% of cells in BM and spleen were donor derived were included in the analysis.
B cell isolation
BM cells were depleted of erythrocytes, then incubated with an antibody cocktail including biotinylated antibodies to CD43, Ter119, CD4, IgD and Gr-1. Cells with bound antibodies were removed by incubation with anti-biotin magnetic beads followed by passage through LS columns (Miltenyi Biotec). Unbound cells were collected. Splenic B cells were similarly isolated but omitting the use of IgD depleting antibodies. The purity of these preparations was >90% in all cases, as determined by B220 and CD19 staining.
PCR assays for RS and Ig gene recombinations
PCR reactions were done in a final volume of 50 μl containing 50, 12.5, 3.1, 0.78 ng of B cell or spleen cell genomic DNA. The Vλ1-to-Jλ1 excision product DNA rearrangements were done using the oligonucleotides and PCR conditions as described (Tiegs et al., 1993). RS-to-IκRS and RS-to-Vκ PCR assays were performed using primers B and C (Retter and Nemazee, 1998) along with a Vκ degenerate primer (Schlissel and Baltimore, 1989). Samples were amplified 25–30 cycles 1 min at 94° C, 1 min at 60°, and 1 min at 72°. PCR products were electrophoresed in 1.5% agarose gels, blotted on nylon membranes (Zeta-Probe membranes, Bio-Rad), and hybridized with radioactive probes as previously described (Aït-Azzouzene et al., 2005). Signals were quantified using a Phosphorimager using ImageQuant software (Molecular Dynamics).
Sequencing of VκlJκ5 light chains
Igλ+ splenocytes were magnetically sorted using anti-Igλ1-3 biotinylated antibody and anti-biotin microbeads (Miltenyi) followed by cell sorting (FACSAria, BD). Bead isolated cells were stained with anti-Igκ (187.1 Alexa 647), CD4 PerCP-Cy5.5, CD3 PerCP-Cy5.5, CD19 PE-Cy7, B220 PacificBlue and streptavidin PE to detect the λ cells. Sorted λ+/κ− B cells were confirmed to be >98% Igλ+. DNeasy kit was used to isolate genomic DNA from ~2 million cells/ sample. Vκ degenerate and Jκ5 primer (5′-TGCCACGTCAACTGATAATGAGCCCTCTCC-3′) were used for PCR amplification of Vκ-Jκ5 rearrangements. The PCR products were electrophoresed on 1% agarose gels, purified using a kit (Qiagen), cloned in PCR4-TOPO plasmid vector (Invitrogen), and the inserts sequenced (Eton Bioscience). Sequence analysis was carried out with the Ig Blast program (http://www.ncbi.nlm.nih.gov/igblast/).
Hybridoma generation and analysis, antibody assays
B220+ spleen cells were cultured for 48 hours in IMDM supplemented with 10% FCS and LPS (50 μg/ml) and fused with the SP2/0 myeloma line using polyethelene glycol. Cells from each fusion were then plated into 96-well plates and hybrids selected using HAT medium (ATCC). Due to the low plating density, no 96-well plate showed growth in more than 15 wells. Any wells with two distinct colonies were excluded. Cell supernatants were then screened for IgMκ or IgMλ and confirmed by intracellular staining for Igλ and Igκ. Enzyme linked immunosorbent assays for antibodies and immunoglobulin levels in culture supernatants or mouse sera were carried out essentially as described (Gavin et al., 2006).
Statistical analysis
Group comparisons were analyzed by 2-tailed Student’s T-test unless otherwise indicated. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors thank S. Kupriyanov and G. Martin of the TSRI Mouse Genetics Core and M. Lim, P. Skog, and H. Kitchel of our laboratory for technical help, A. Gavin, L. Verkoczy and R. Rickert for suggestions, D. Huzsar for Jκ-Cκ deleted mice and A. Harris and A. Strasser for E2-22 Bcl2Tg mice and Dr. D. Kono for anti-dsDNA control serum. This work was supported by RO1AI33608 to DN. J.L.V. was supported by training grant T32AI007606 and an NIH/NRSA Predoctoral Fellowship T31AI052484.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aït-Azzouzene D, Verkoczy L, Peters J, Gavin A, Skog P, Vela JL, Nemazee D. An immunoglobulin C{kappa}-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. JExp Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel TW, Scherer A, Adachi T, Auch D, Ayane M, Reth M. The ribose 5-phosphate isomerase-encoding gene is located immediately downstream from that encoding murine immunoglobulin kappa. Gene. 1995;156:191–197. doi: 10.1016/0378-1119(94)00901-4. [DOI] [PubMed] [Google Scholar]
- Berg J, McDowell M, Jack HM, Wabl M. Immunoglobulin lambda gene rearrangement can precede kappa gene rearrangement. Dev Immunol. 1990;1:53–57. doi: 10.1155/1990/56014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauninger A, Goossens T, Rajewsky K, Kuppers R. Regulation of immunoglobulin light chain gene rearrangements during early B cell development in the human. Eur J Immunol. 2001;31:3631–3637. doi: 10.1002/1521-4141(200112)31:12<3631::aid-immu3631>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- Casellas R, Zhang Q, Zheng NY, Mathias MD, Smith K, Wilson PC. Igkappa allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–160. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- Chen J, Trounstine M, Kurahara C, Young F, Kuo CC, Xu Y, Loring JF, Alt FW, Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993;12:821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire . Nature. 1994;371:389–395. doi: 10.1038/371389a0. [see comments] [DOI] [PubMed] [Google Scholar]
- Daitch LE, Moore MW, Persiani DM, Durdik JM, Selsing E. Transcription and recombination of the murine RS element. JImmunol. 1992;149:832–840. [PubMed] [Google Scholar]
- Diaw L, Siwarski D, DuBois W, Jones G, Huppi K. Double producers of kappa and lambda define a subset of B cells in mouse plasmacytomas. Mol Immunol. 2000;37:775–781. doi: 10.1016/s0161-5890(00)00100-0. [DOI] [PubMed] [Google Scholar]
- Dunda O, Corcos D. Recombining sequence recombination in normal kappa-chain-expressing B cells. JImmunol. 1997;159:4362–4366. [PubMed] [Google Scholar]
- Durdik J, Moore MW, Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984;307:749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Feddersen RM, Van Ness BG. Double recombination of a single immunoglobulin kappa-chain allele: implications for the mechanism of rearrangement. Proc Natl Acad Sci USA. 1985;82:4793–4797. doi: 10.1073/pnas.82.14.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, Padovan E, Lanzavecchia A. kappa+lambda+ dual receptor B cells are present in the human peripheral repertoire. JExp Med. 1995;181:1245–1250. doi: 10.1084/jem.181.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollahon KA, Hagman J, Brinster RL, Storb U. Ig lambda-producing B cells do not show feedback inhibition of gene rearrangement. JImmunol. 1988;141:2771–2780. [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Mason DY, Jorgensen H, Brink RA, Pritchard-Briscoe H, Loughnan M, et al. Clonal silencing of self-reactive B lymphocytes in a transgenic mouse model. Cold Spring Harb Symp Quant Biol. 1989;54:907–20. doi: 10.1101/sqb.1989.054.01.106. [DOI] [PubMed] [Google Scholar]
- Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Inlay M, Alt FW, Baltimore D, Xu Y. Essential roles of the kappa light chain intronic enhancer and 3′ enhancer in kappa rearrangement and demethylation. Nat Immunol. 2002;3:463–468. doi: 10.1038/ni790. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [see comments] [DOI] [PubMed] [Google Scholar]
- Klein F, Feldhahn N, Mooster JL, Sprangers M, Hofmann WK, Wernet P, Wartenberg M, Muschen M. Tracing the pre-B to immature B cell transition in human leukemia cells reveals a coordinated sequence of primary and secondary IGK gene rearrangement, IGK deletion, and IGL gene rearrangement. JImmunol. 2005;174:367–375. doi: 10.4049/jimmunol.174.1.367. [DOI] [PubMed] [Google Scholar]
- Klobeck HG, Zachau HG. The human CK gene segment and the kappa deleting element are closely linked. Nucleic Acids Res. 1986;14:4591–4603. doi: 10.1093/nar/14.11.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. JExp Med. 1997;186:1513–1522. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. JExp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Louzoun Y, Weigert M. Editing Anti-DNA B Cells by V{lambda}x. JExp Med. 2004;199:337–346. doi: 10.1084/jem.20031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Durdik J, Persiani DM, Selsing E. Deletions of kappa chain constant region genes in mouse lambda chain-producing B cells involve intrachromosomal DNA recombinations similar to V-J joining. Proc Natl Acad Sci USA. 1985;82:6211–6215. doi: 10.1073/pnas.82.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. JImmunol. 1997;158:6019–28. [PubMed] [Google Scholar]
- Muller B, Reth M. Ordered activation of the Ig lambda locus in Abelson B cell lines. JExp Med. 1988;168:2131–2137. doi: 10.1084/jem.168.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Stappert H, Reth M. A physical map and analysis of the murine C kappa-RS region show the presence of a conserved element. Eur JImmunol. 1990;20:1409–1411. doi: 10.1002/eji.1830200631. [DOI] [PubMed] [Google Scholar]
- Nadel B, Cazenave PA, Sanchez P. Murine lambda gene rearrangements: the stochastic model prevails over the ordered model. EMBO J. 1990;9:435–440. doi: 10.1002/j.1460-2075.1990.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Papavasiliou F, Jankovic M, Nussenzweig MC. Surrogate or conventional light chains are required for membrane immunoglobulin mu to activate the precursor B cell transition. JExp Med. 1996;184:2025–2030. doi: 10.1084/jem.184.5.2025. [published erratum appears in J Exp Med 1997 Jan 6;185(1):183] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou F, Misulovin Z, Suh H, Nussenzweig MC. The role of Ig beta in precursor B cell transition and allelic exclusion. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- Pauza ME, Rehmann JA, LeBien TW. Unusual patterns of immunoglobulin gene rearrangement and expression during human B cell ontogeny: human B cells can simultaneously express cell surface kappa and lambda light chains. JExp Med. 1993;178:139–149. doi: 10.1084/jem.178.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- Persiani DM, Durdik J, Selsing E. Active lambda and kappa antibody gene rearrangement in Abelson murine leukemia virus-transformed pre-B cell lines. JExp Med. 1987;165:1655–1674. doi: 10.1084/jem.165.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden DA, Wu GE. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci USA. 1991;88:10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. JExp Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Selsing E, Daitch LE. Immunoglobulin λ genes. In: Honjo T, Alt F, editors. Immunoglubulin Genes. London: Acadenic Press Limited; 1995. pp. 194–203. [Google Scholar]
- Shapiro MA, Weigert M. How immunoglobulin V kappa genes rearrange. JImmunol. 1987;139:3834–3839. [PubMed] [Google Scholar]
- Shimizu T, Iwasato T, Yamagishi H. Deletions of immunoglobulin C kappa region characterized by the circular excision products in mouse splenocytes. JExp Med. 1991;173:1065–1072. doi: 10.1084/jem.173.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch KA, Bakhshi A, Goldman P, Korsmeyer SJ. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 1985;316:260–262. doi: 10.1038/316260a0. [DOI] [PubMed] [Google Scholar]
- Siminovitch KA, Moore MW, Durdik J, Selsing E. The human kappa deleting element and the mouse recombining segment share DNA sequence homology. Nucleic Acids Res. 1987;15:2699–2705. doi: 10.1093/nar/15.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanopoulou E, Roman CA, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzweig MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes & Development. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM, Cory S. Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Current Topics in Microbiology & Immunology. 1990;166:175–81. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Zou YR, Bluethmann H, Kitamura D, Muller U, Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO J. 1993;12:2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. JExp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, Kraus M, Hardy RR, Schlissel MS, Rajewsky K, Behrens TW. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Ait-Azzouzene D, Skog P, Martensson A, Lang J, Duong B, Nemazee D. A role for nuclear factor kappa B/rel transcription factors in the regulation of the recombinase activator genes. Immunity. 2005;22:519–531. doi: 10.1016/j.immuni.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li H, Suri-Payer E, Hardy RR, Weigert M. Regulation of anti-DNA B cells in recombination-activating gene-deficient mice. J Exp Med. 1998;188:1247–1254. doi: 10.1084/jem.188.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Davidson L, Alt FW, Baltimore D. Deletion of the Ig kappa light chain intronic enhancer/matrix attachment region impairs but does not abolish V kappa J kappa rearrangement. Immunity. 1996;4:377–385. doi: 10.1016/s1074-7613(00)80251-4. [DOI] [PubMed] [Google Scholar]
- Young F, Ardman B, Shinkai Y, Lansford R, Blackwell TK, Mendelsohn M, Rolink A, Melchers F, Alt FW. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes & Development. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [published erratum appears in Genes Dev 1995 Dec 15;9(24):3190] [DOI] [PubMed] [Google Scholar]
- Zou YR, Takeda S, Rajewsky K. Gene targeting in the Ig kappa locus: efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. EMBO J. 1993;12:811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.