Abstract
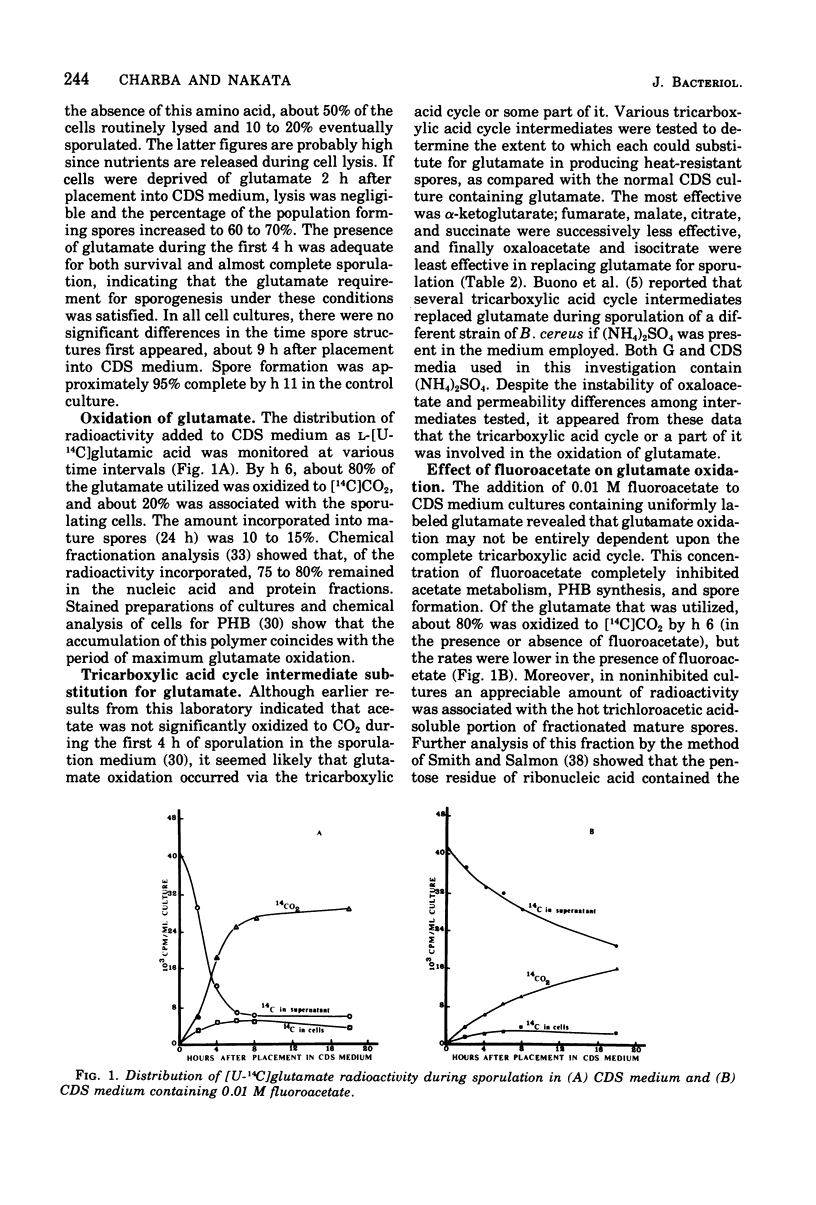
Bacillus cereus T, sporulating in a chemically defined medium under optimum conditions, requires substrate quantities of glutamate during the first 4 h of sporogenesis. Seventy percent of the glutamate utilized was catabolized to CO2 during this period, with the remaining glutamate carbon assimilated into various spore constituents, principally protein and nucleic acid. The importance of glutamate as the primary source of reducing potential and energy for early stages of spore formation was investigated. Although the relative efficiency at which tricarboxylic acid cycle intermediates substituted for glutamate was suggestive of oxidation via the tricarboxylic acid cycle, only partial inhibition of glutamate oxidation by fluoroacetate was observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R., HALVORSON H., CHURCH B. Intermediate metabolism of aerobic spores. III. The mechanism of glucose and hexose phosphate oxidation in extracts of Bacillus cereus spores. J Bacteriol. 1959 Jan;77(1):43–54. doi: 10.1128/jb.77.1.43-54.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesterhaft M. D., Freese E. Role of pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and malic enzyme during growth and sporulation of Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6062–6070. [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARY N. D., BARD R. C. Effect of nutrition on the growth and metabolism of Bacillus subtilis. J Bacteriol. 1952 Oct;64(4):501–512. doi: 10.1128/jb.64.4.501-512.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. PATHWAYS OF GLUCOSE CATABOLISM IN BACILLUS CEREUS. J Bacteriol. 1964 Feb;87:377–386. doi: 10.1128/jb.87.2.377-386.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRELET N. Nutrition azotée et sporulation de Bacillus cereus var. mycoides. Ann Inst Pasteur (Paris) 1955 Jan;88(1):60–75. [PubMed] [Google Scholar]
- HANSON H. M., WITOSLAWSKI J. J., CAMPBELL E. H. REVERSIBLE DISRUPTION OF A WAVELENGTH DISCRIMINATION IN PIGEONS FOLLOWING ADMINISTRATION OF PHENIPRAZINE. Toxicol Appl Pharmacol. 1964 Nov;6:690–695. doi: 10.1016/0041-008x(64)90119-x. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG M. M., SHEN S. C., BRAUNSTEIN A. E. Distribution of L-alanine dehydrogenase and L-glutamate dehydrogenase in Bacilli. Biochim Biophys Acta. 1959 Nov;36:288–289. doi: 10.1016/0006-3002(59)90111-8. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNAN A., STRECKER H. J., WAELSCH H. Glutamine, glutamic acid, and glycolysis in Bacillus subtilis. J Biol Chem. 1954 Dec;211(2):883–891. [PubMed] [Google Scholar]
- Kennedy R. S., Malveaux F. J., Cooney J. J. Effects of glutamic acid on sporulation of Bacillus cereus and on spore properties. Can J Microbiol. 1971 Apr;17(4):511–519. doi: 10.1139/m71-085. [DOI] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The crystallization of fumarase. Biochem J. 1952 Jul;51(4):490–494. doi: 10.1042/bj0510490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMICK N. G., HALVORSON H. O. PURIFICATION AND PROPERTIES OF L-ALANINE DEHYDROGENASE FROM VEGETATIVE CELLS OF BACILLUS CEREUS. J Bacteriol. 1964 Jan;87:68–74. doi: 10.1128/jb.87.1.68-74.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLET J., AUBERT J. P. [The metabolism of glutamic acid in the course of sporulation in Bacillus megaterium]. Ann Inst Pasteur (Paris) 1960 Feb;98:282–290. [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H. M. Role of acetate in sporogenesis of Bacillus cereus. J Bacteriol. 1966 Feb;91(2):784–788. doi: 10.1128/jb.91.2.784-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., De Pinto J., Bulla L. A., Jr Sporulation of Bacillus thuringiensis without concurrent derepression of the tricarboxylic acid cycle. J Bacteriol. 1974 Jan;117(1):321–323. doi: 10.1128/jb.117.1.321-323.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Reysset G., Aubert J. P. Relationship between sporulation and mutations impairing glutamine synthetase in Bacillus megaterium. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1237–1241. doi: 10.1016/s0006-291x(75)80362-7. [DOI] [PubMed] [Google Scholar]
- SMITH R. C., SALMON W. D. EFFECT OF ETHIONINE ON THE RIBONUCLEIC ACID, DEOXYRIBONUCLEIC ACID, AND PROTEIN CONTENT OF ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:687–692. doi: 10.1128/jb.89.3.687-692.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. M. Role of tricarboxylic acid cycle in bacterial sporulation. Biochem Biophys Res Commun. 1970 May 22;39(4):651–654. doi: 10.1016/0006-291x(70)90254-8. [DOI] [PubMed] [Google Scholar]
- West D. J., Tuveson R. W., Barratt R. W., Fincham J. R. Allosteric effects in nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase from Neurospora. J Biol Chem. 1967 May 10;242(9):2134–2138. [PubMed] [Google Scholar]


