Abstract
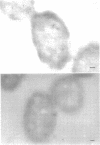
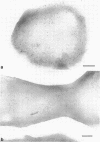
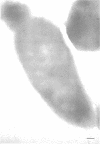
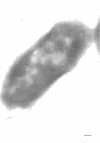
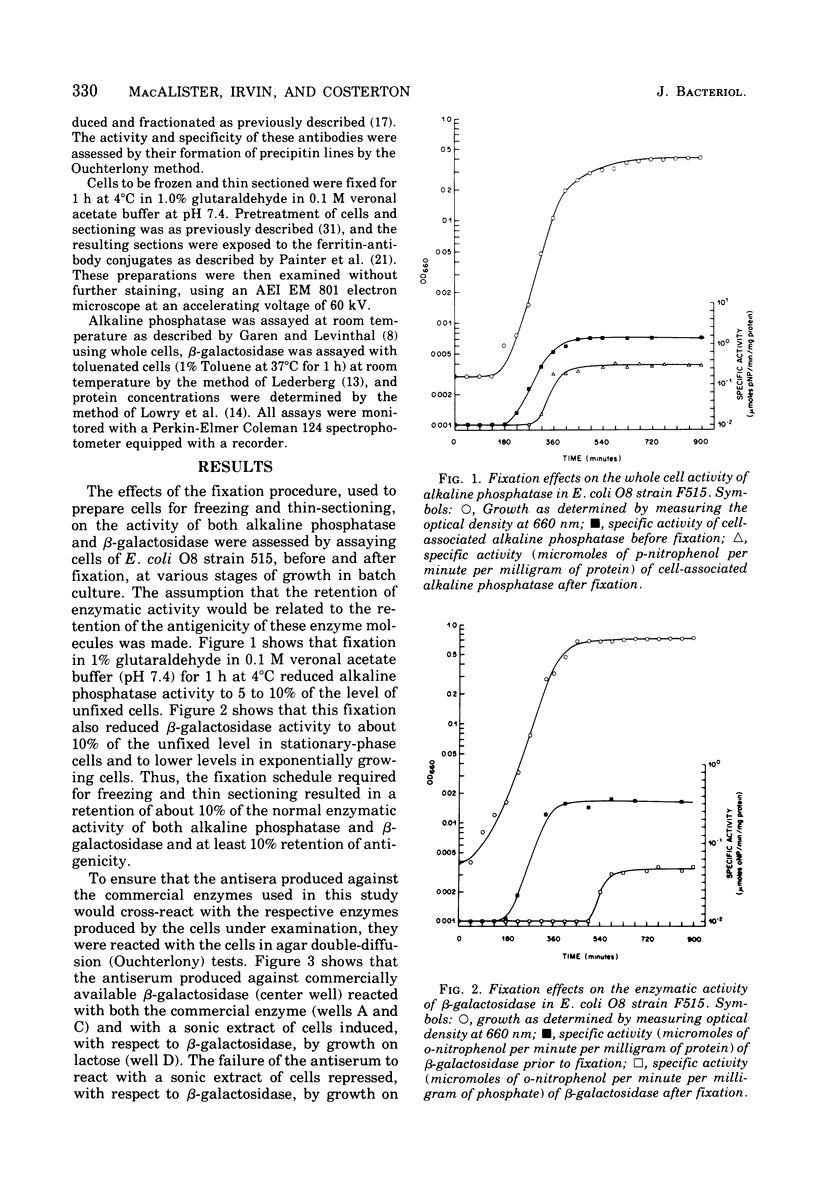
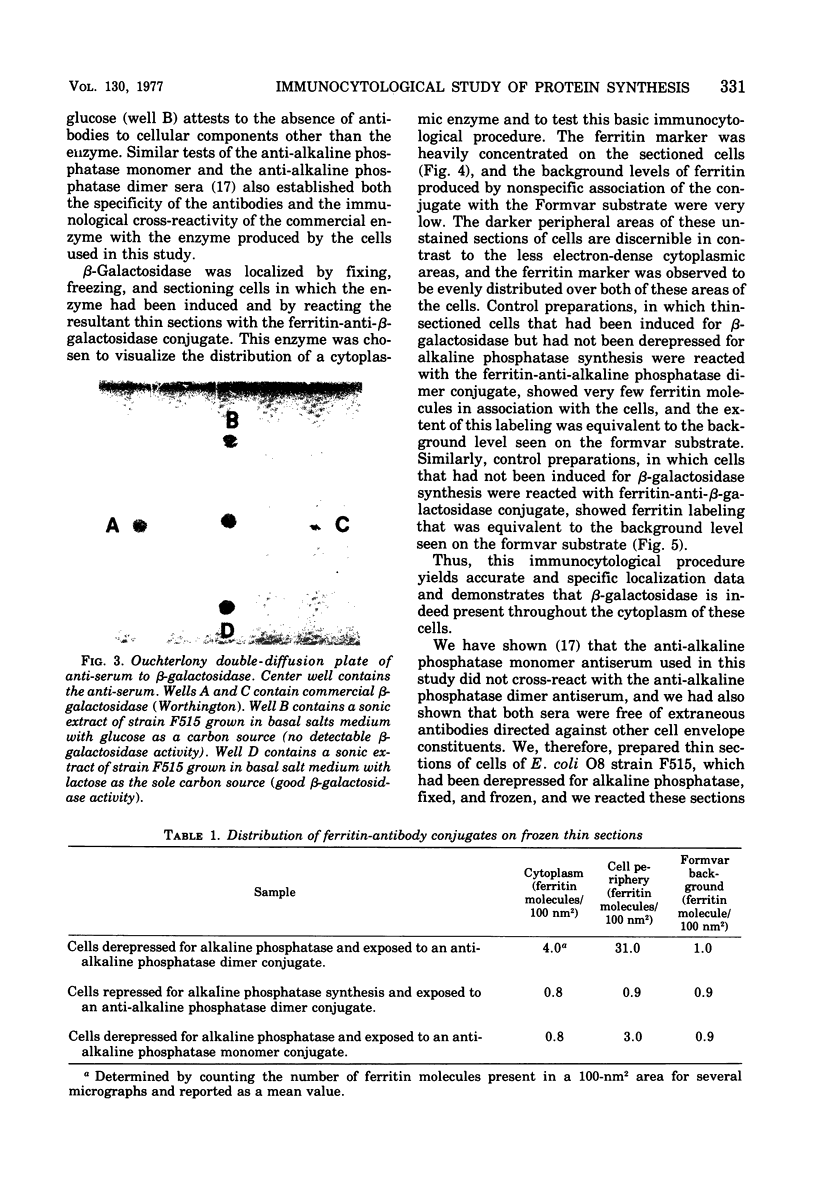
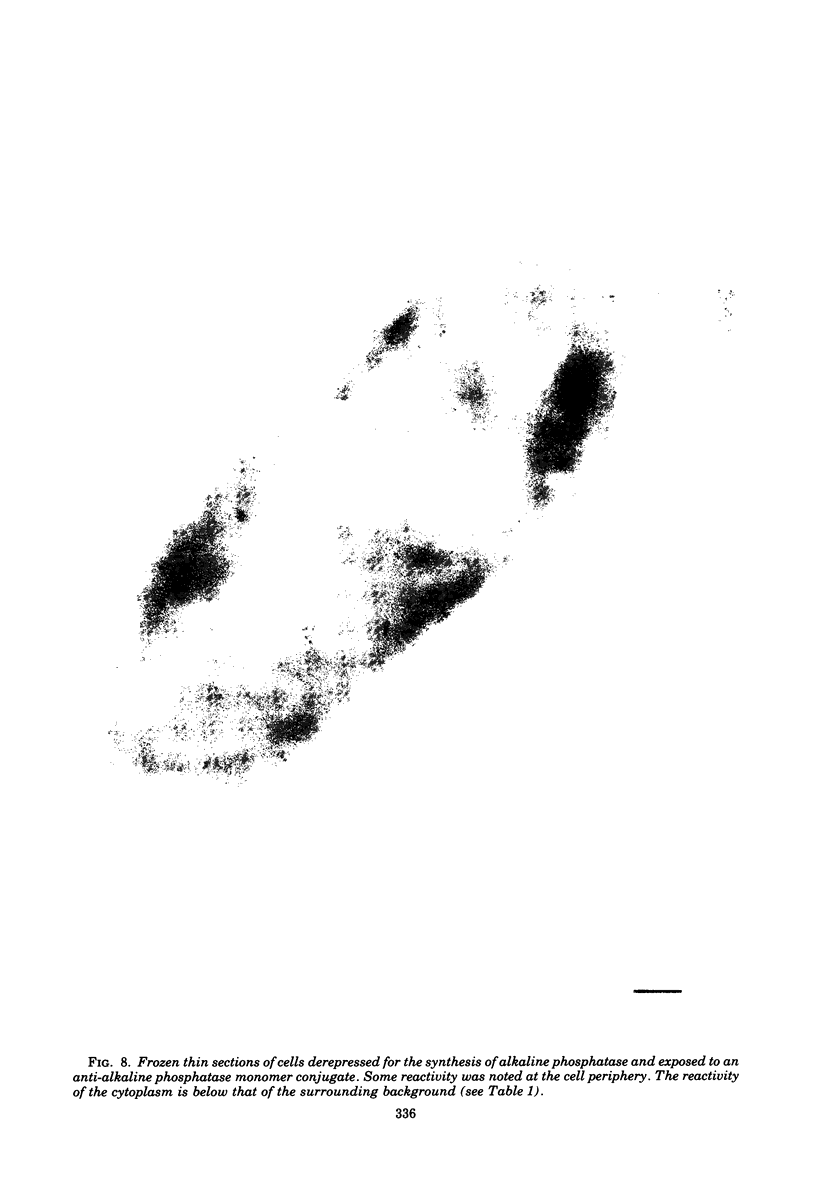
Ferritin-conjugated specific antibodies have been used to localize beta-galactosidase and both the monomer and active dimer of alkaline phosphatase in frozen thin sections of cells of Escherichia coli O8 strain F515. The even distribution of the ferritin marker throughout cells that had been induced for beta-galactosidase synthesis, frozen, sectioned, and exposed to ferritin-anti-beta-galactosidase conjugate showed that this enzyme was present throughout the cytoplasm of these cells. Frozen thin sections of cells that had been derepressed for the synthesis of alkaline phosphatase were exposed to both ferritin-anti-alkaline phosphatase monomer and ferritin-anti-alkaline phosphatase dimer conjugates, and the ferritin markers showed a peripheral distribution of both the monomer and the dimer of this enzyme. This indicates that alkaline phosphatase is present only in the peripheral regions of the cell and argues against the existence of a cytoplasmic pool of inactive monomers of this enzyme. This peripheral location of both the monomers and dimers of alkaline phosphatase supports the developing concensus that this enzyme is, like other wall-associated enzymes, synthesized in association with the cytoplasmic membrane and vectorially transported to the periplasmic area, where it assumes its tertiary and quaternary structure and acquires its enzymatic activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Localization of polyribosomes containing alkaline phosphatase nascent polypeptides on membranes of Escherichia coli. J Bacteriol. 1974 Jan;117(1):290–301. doi: 10.1128/jb.117.1.290-301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. Intracellular distribution of ribosomes and polyribosomes in Bacillus megaterium. J Mol Biol. 1970 Sep 28;52(3):467–481. doi: 10.1016/0022-2836(70)90413-4. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Synthesis, assembly, and localization of periplasmic cytochrome c. J Biol Chem. 1972 Sep 25;247(18):5935–5943. [PubMed] [Google Scholar]
- Glick M. C., Warren L. Membranes of animal cells. 3. Amino acid incorporation by isolated surface membranes. Proc Natl Acad Sci U S A. 1969 Jun;63(2):563–570. doi: 10.1073/pnas.63.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975 Apr;122(1):34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. The beta-d-galactosidase of Escherichia coli, strain K-12. J Bacteriol. 1950 Oct;60(4):381–392. doi: 10.1128/jb.60.4.381-392.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacaAlister T. J., Irvin R. T., Costerton J. W. Cell surface-localized alkaline phosphatase of Escherichia coli as visualized by reaction product deposition and ferritin-labeled antibodies. J Bacteriol. 1977 Apr;130(1):318–328. doi: 10.1128/jb.130.1.318-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M., Michaels A. Ribosomes bound to chloroplast membranes in Chlamydomonas reinhardtii. J Cell Biol. 1974 Jan;60(1):65–77. doi: 10.1083/jcb.60.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K., ROBERTS R. B. The utilization of acetate for synthesis in Escherichia coli. J Biol Chem. 1954 Mar;207(1):81–95. [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Tokuyasu K. T., Singer S. J. Immunoferritin localization of intracellular antigens: the use of ultracryotomy to obtain ultrathin sections suitable for direct immunoferritin staining. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1649–1653. doi: 10.1073/pnas.70.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- Redman C. M. Studies on the transfer of incomplete polypeptide chains across rat liver microsomal membranes in vitro. J Biol Chem. 1967 Feb 25;242(4):761–768. [PubMed] [Google Scholar]
- Rouvière J., Lederberg S., Granboulan P., Gros F. Structural sites of RNA synthesis in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):413–430. doi: 10.1016/0022-2836(69)90185-5. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- Sanders R. L., May B. K. Evidence for extrusion of unfolded extracellular enzyme polypeptide chains through membranes of Bacillus amyloliquefaciens. J Bacteriol. 1975 Sep;123(3):806–814. doi: 10.1128/jb.123.3.806-814.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Studies on R mutants with an incomplete core, derived from E. coli O8:K27. Eur J Biochem. 1970 Oct;16(2):382–392. doi: 10.1111/j.1432-1033.1970.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani A. Alkaline phosphatase subunits and their dimerization in vivo. J Bacteriol. 1968 Oct;96(4):1200–1207. doi: 10.1128/jb.96.4.1200-1207.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]