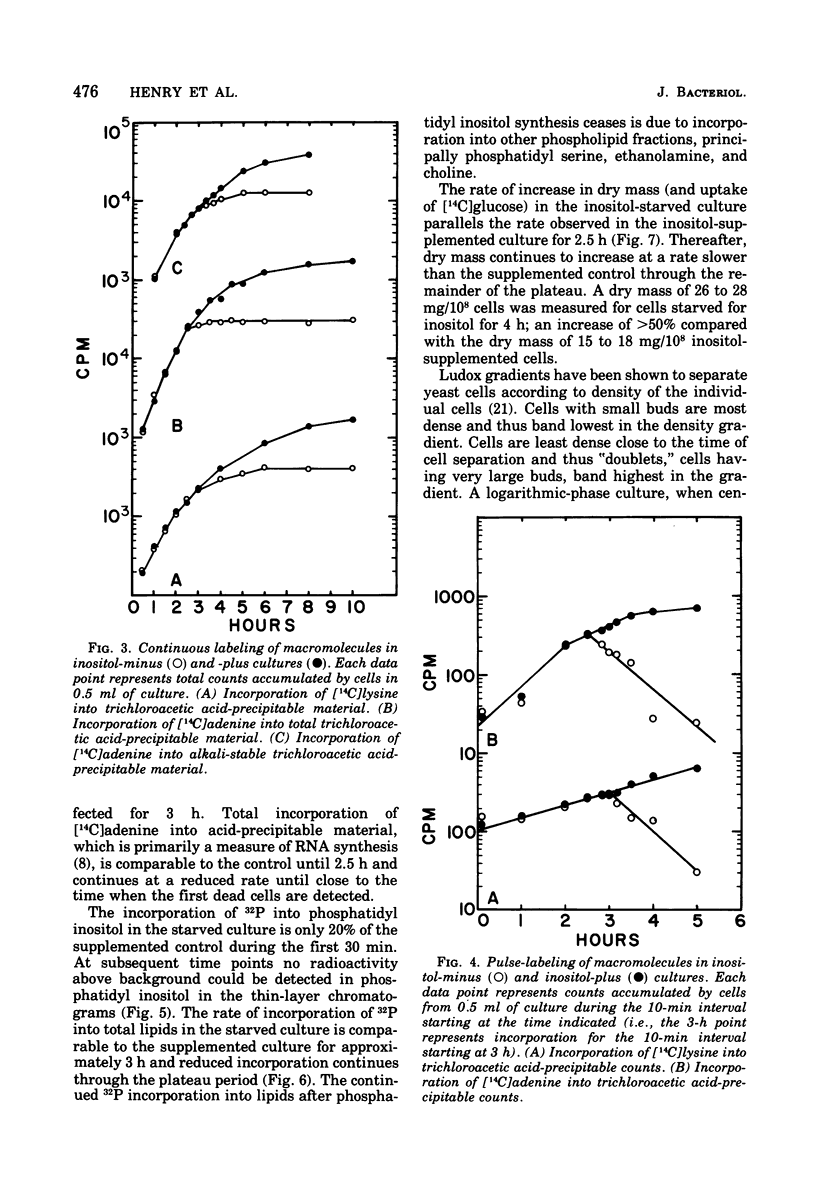
Abstract
Upon starvation for inositol, a phospholipid precursor, an inositol-requiring mutant of Saccharomyces cerevisiae has been shown to die if all other conditions are growth supporting. The growth and metabolism of inositol-starved cells has been investigated in order to determine the physiological state leading to "inositolless death". The synthesis of the major inositol-containing phospholipid ceases within 30 min after the removal of inositol from the growth medium. The cells, however, continue in an apparently normal fashion for one generation (2 h under the growth conditions used in this study). The cessation of cell division is not preceded or accompanied by any detectable change in the rate of macromolecular synthesis. When cell division ceases, the cells remain constant in volume, whereas macromolecular synthesis continues at first at an unchanged rate and eventually at a decreasing rate. Macromolecular synthesis terminates after about 4 h of inositol starvation, at approximately the time when the cells begin to die. Cell death is also accompanied by a decline in cellular potassium and adenosine triphosphate levels. The cells can be protected from inositolless death by several treatments that block cellular metabolism. It is concluded that inositol starvation results in a imbalance between the expansion of cell volume and the accumulation of cytoplasmic constituents. This imbalance is very likely the cause of inositolless death.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brendel M., Langjahr U. G. "Thymineless death" in a strain of Saccharomyces cerevisiae auxotrophic for deoxythymidine-5'-monophosphate. Mol Gen Genet. 1974;131(4):351–358. doi: 10.1007/BF00264865. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P., HARSCH M., LAMPEN J. O. ACTION OF THE POLYENE ANTIBIOTICS FILIPIN, NYSTATIN AND N-ACETYLCANDIDIN ON THE YEAST CELL MEMBRANE. J Gen Microbiol. 1964 May;35:249–259. doi: 10.1099/00221287-35-2-249. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., ARMSTRONG W. M. The total intracellular concentration of solutes in yeast and other plant cells and the distensibility of the plant-cell wall. Biochem J. 1961 Dec;81:631–639. doi: 10.1042/bj0810631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M. S., Weiss B., Costa E. Microassay of adenosine-3',5'-monophosphate (cyclic AMP) in brain and other tissues by the luciferin-luciferase system. J Neurochem. 1971 Feb;18(2):183–192. doi: 10.1111/j.1471-4159.1971.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Getz G. S., Jakovcic S., Heywood J., Frank J., Rabinowitz M. A two-dimensional thin-layer chromatographic system for phospholipid separation. The analysis of yeast phospholipids. Biochim Biophys Acta. 1970 Dec 15;218(3):441–452. doi: 10.1016/0005-2760(70)90007-x. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A. 1970 Jun;66(2):352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D., Snipes W. Changes in the restriction of molecular rotational diffusion of water-soluble spin labels during fatty acid starvation of yeast. Biophys J. 1976 Jun;16(6):641–653. doi: 10.1016/S0006-3495(76)85718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. L., Steiner M. R. The occurrence of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. J Biol Chem. 1968 Sep 25;243(18):4889–4893. [PubMed] [Google Scholar]
- Letters R. Phospholipids of yeast. II. Extraction, isolation and characterisation of yeast phospholipids. Biochim Biophys Acta. 1966 Jun 1;116(3):489–499. [PubMed] [Google Scholar]
- Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science. 1966 Jan 7;151(3706):86–88. doi: 10.1126/science.151.3706.86. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- STRAUSS B. S. Cell death and unbalanced growth in Neurospora. J Gen Microbiol. 1958 Jun;18(3):658–669. doi: 10.1099/00221287-18-3-658. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. 3. An inositol requirement for the function of the glucose active transport system. Biochem Biophys Res Commun. 1971 Jun 4;43(5):968–975. doi: 10.1016/0006-291x(71)90557-2. [DOI] [PubMed] [Google Scholar]
- Shulman R. W., Hartwell L. H., Warner J. R. Synthesis of ribosomal proteins during the yeast cell cycle. J Mol Biol. 1973 Feb 5;73(4):513–525. doi: 10.1016/0022-2836(73)90097-1. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Debusk A. G. Inositol-less death in Neurospora and cellular ageing. Nat New Biol. 1973 May 16;243(124):72–74. [PubMed] [Google Scholar]




