Abstract
Cell of Spirochaeta aurantia M1 suspended in isotropic buffer solution swam in nearly straight lines and appeared to spin around their longitudinal axis. Occasionally, cells stopped and flexed, and then resumed translational motility, usually in a different direction. The average cell velocity was 26 micron/s. A quantitative assay for chemotaxis was used to test various chemicals for their ability to attract S. aurantia M1. The cells exhibited a tactic response toward 5 X 10(-2) M D-glucose between 10 and 35degree C; the optimum response was at 25degree C. At 5 degree C motility was not impaired, but D-glucose taxis was abolished. Chemotaxis toward D-glucose was stimulated by L-cysteine (2 X 10(-4) M). D-Glucose, 2-deoxy-D-glucose, alpha-methyl-D-glucoside, D-galactose, D-fucose, D-mannose, D-fructose, D-xylose, maltose, cellobiose, and D-glucosamine were effectve attractants for S. aurantia M1. D-Galactose taxis and D-fucose taxis were induced by the presence of D-galactose in the growth medium. The amino acids tested did not serve as attractants, tgrowing cells of S. aurantia M1 exhibited an aerotactic response.
Full text
PDF


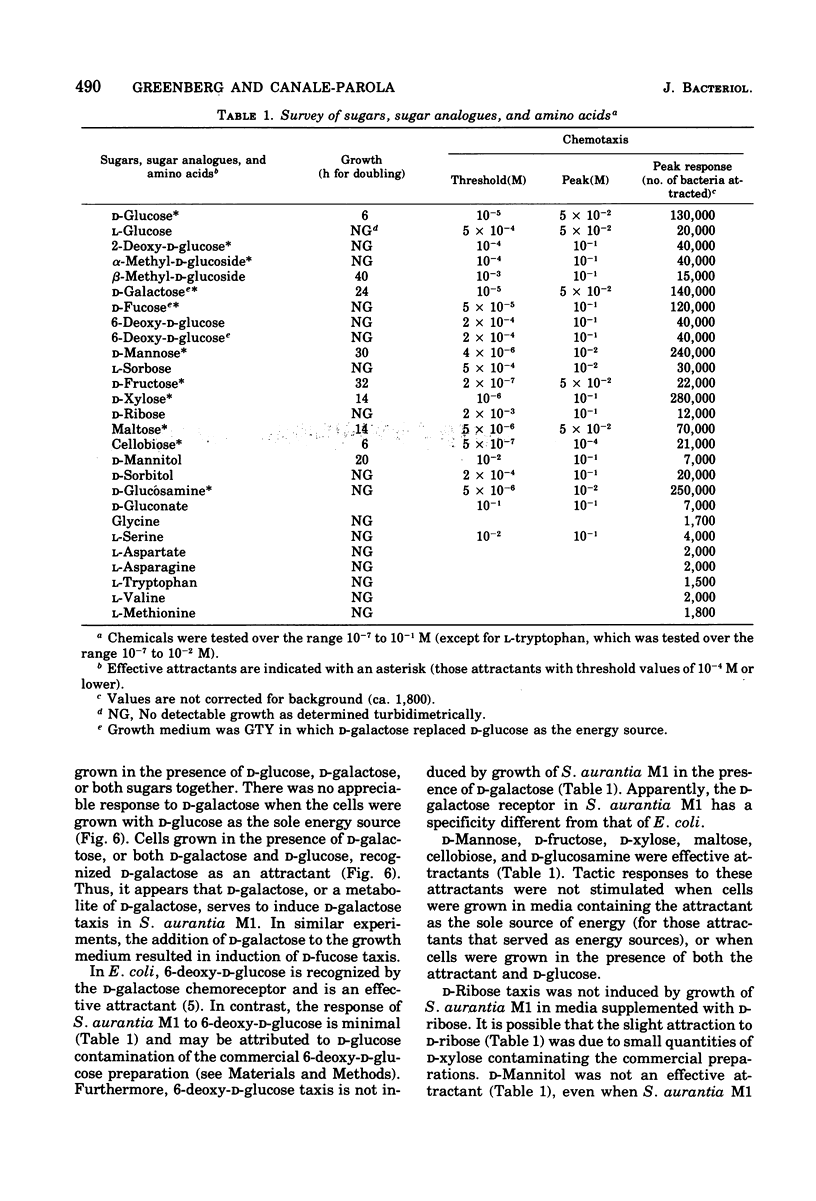
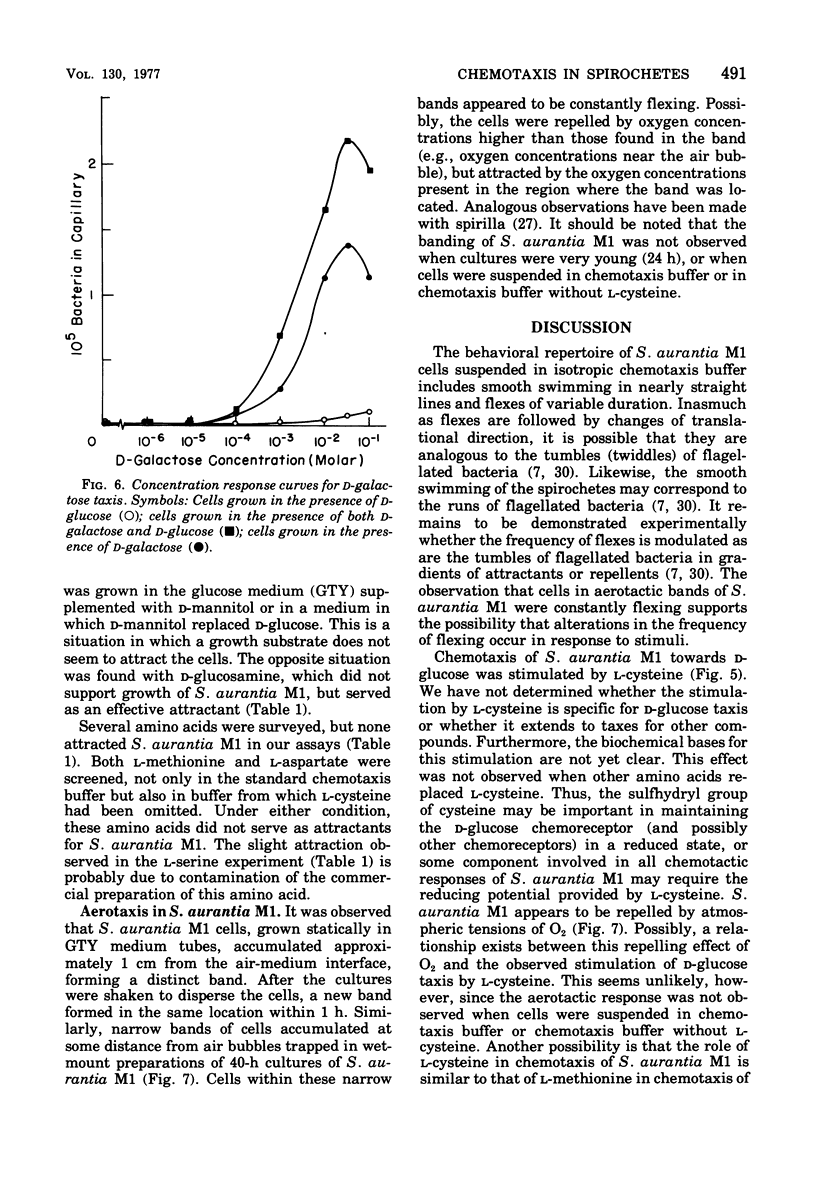



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Adler J., Dahl M. M. A method for measuring the motility of bacteria and for comparing random and non-random motility. J Gen Microbiol. 1967 Feb;46(2):161–173. doi: 10.1099/00221287-46-2-161. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Bharier M. A., Eiserling F. A., Rittenberg S. C. Eletron microscopic observations on the structure of Treponema zuelzerae and its axial filaments. J Bacteriol. 1971 Jan;105(1):413–421. doi: 10.1128/jb.105.1.413-421.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Morphological and ecological characteristics of Spirochaeta plicatilis. Arch Mikrobiol. 1973;89(4):273–289. doi: 10.1007/BF00408895. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Morphology and physiology of Spirochaeta aurantia strains isolated from aquatic habitats. Arch Microbiol. 1975 Sep 30;105(1):1–12. doi: 10.1007/BF00447104. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E., Holt S. C., Udris Z. Isolation of free-living, anaerobic spirochetes. Arch Mikrobiol. 1967;59(1):41–48. doi: 10.1007/BF00406315. [DOI] [PubMed] [Google Scholar]
- Cox P. J., Twigg G. I. Leptospiral motility. Nature. 1974 Jul 19;250(463):260–261. doi: 10.1038/250260a0. [DOI] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to Plant Root Exudates. Plant Physiol. 1976 May;57(5):820–823. doi: 10.1104/pp.57.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W., Lovely P., Koshland D. E., Jr Quantitative analysis of bacterial migration in chemotaxis. Nat New Biol. 1972 Mar 29;236(65):120–123. doi: 10.1038/newbio236120a0. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Spirochaeta halophila sp. n., a facultative anaerobe from a high-salinity pond. Arch Microbiol. 1976 Nov 2;110(23):185–194. doi: 10.1007/BF00690227. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Canale-Parola E. Fine structure of Spirochaeta stenostrepta, a free-living, anaerobic spirochete. J Bacteriol. 1968 Sep;96(3):822–835. doi: 10.1128/jb.96.3.822-835.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen K. H., Birch-Andersen A. Electron microscopy of endoflagella and microtubules in Treponema reiter. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):37–50. doi: 10.1111/j.1699-0463.1971.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Jackson S., Black S. H. Ultrastructure of Treponema pallidum Nichols following lysis by physical and chemical methods. II. Axial filaments. Arch Mikrobiol. 1971;76(4):325–340. doi: 10.1007/BF00408529. [DOI] [PubMed] [Google Scholar]
- Joseph R., Canale-Parola E. Axial fibrils of anaerobic spirochetes: ultrastructure and chemical characteristics. Arch Mikrobiol. 1972;81(2):146–168. doi: 10.1007/BF00412325. [DOI] [PubMed] [Google Scholar]
- Kaiser G. E., Doetsch R. N. Letter: Enhanced translational motion of Leptospira in viscous environments. Nature. 1975 Jun 19;255(5510):656–657. doi: 10.1038/255656a0. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg N. R. Biology of the chemoheterotrophic spirilla. Bacteriol Rev. 1976 Mar;40(1):55–115. doi: 10.1128/br.40.1.55-115.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. R., Doetsch R. N. Effect of viscosity on bacterial motility. J Bacteriol. 1974 Feb;117(2):696–701. doi: 10.1128/jb.117.2.696-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Kort E. N., Larsen S. H., Ordal G. W., Reader R. W., Adler J. Role of methionine in bacterial chemotaxis: requirement for tumbling and involvement in information processing. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4640–4644. doi: 10.1073/pnas.72.11.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Drift C., De Jong M. H. Chemotaxis toward amino acids in Bacillus subtilis. Arch Mikrobiol. 1974 Mar 4;96(2):83–92. [PubMed] [Google Scholar]
- Wachter M. S., Johnson R. C. Treponeme outer envelope: chemical analysis. Proc Soc Exp Biol Med. 1976 Jan;151(1):97–100. doi: 10.3181/00379727-151-39151. [DOI] [PubMed] [Google Scholar]
- de Jong M. H., van der Drift C., Vogels G. D. Receptors for chemotaxis in Bacillus subtilis. J Bacteriol. 1975 Sep;123(3):824–827. doi: 10.1128/jb.123.3.824-827.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Drift C., Duiverman J., Bexkens H., Krijnen A. Chemotaxis of a motile Streptococcus toward sugars and amino acids. J Bacteriol. 1975 Dec;124(3):1142–1147. doi: 10.1128/jb.124.3.1142-1147.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]