Abstract
Type 1 pili of Escherichia coli are the prototype of the somatic class of pili found on many strains of bacteria. As a first step in the genetic analysis of type 1 piliation, an extensive series of nonpiliated derivatives of E. coli K-12 strain AW405, was characterized to produce attached or free pili when examined in the antiserum or appeared to produce attached or free pili when examined in the electron microscope. The derivatives fell into two classes; phase variants and mutants. Phase variants that formed colonies of two distinctive types, one associated with a predominantly piliated (P+), and the other associated with a nonpiliated (P-) phase, were obtained. Each phase could give rise to the other at a relatively high rate, which was greater in the P- to P+ direction during culture in unshaken liquid medium. In addition, 77 Pil- mutants were selected on the basis of a subtle difference in colonial morphology. The mutants reverted, if at all, at a much lower rate than that of the P- to P+ change. The stability of Pil- derivatives grown in unshaken liquid medium was used as a criterion for distinguishing between phase variants and mutants, Phase variation also effected colonial morphology and chemotactic swarming. These properties did not directly depend upon piliation since Pil- mutants were only slightly altered in colonial form and unaltered in chemotactic swarming. Piliation of Pil+ bacteria was quantitatively affected by growth conditions.
Full text
PDF


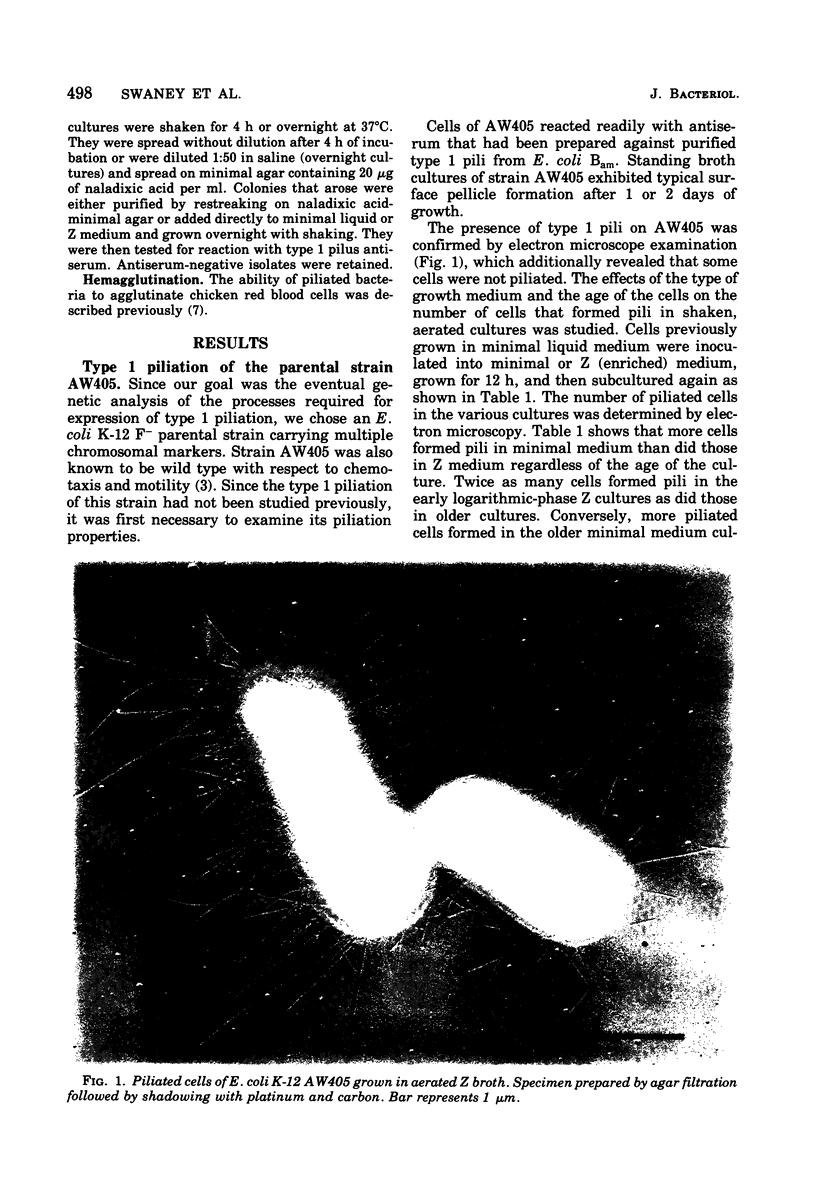


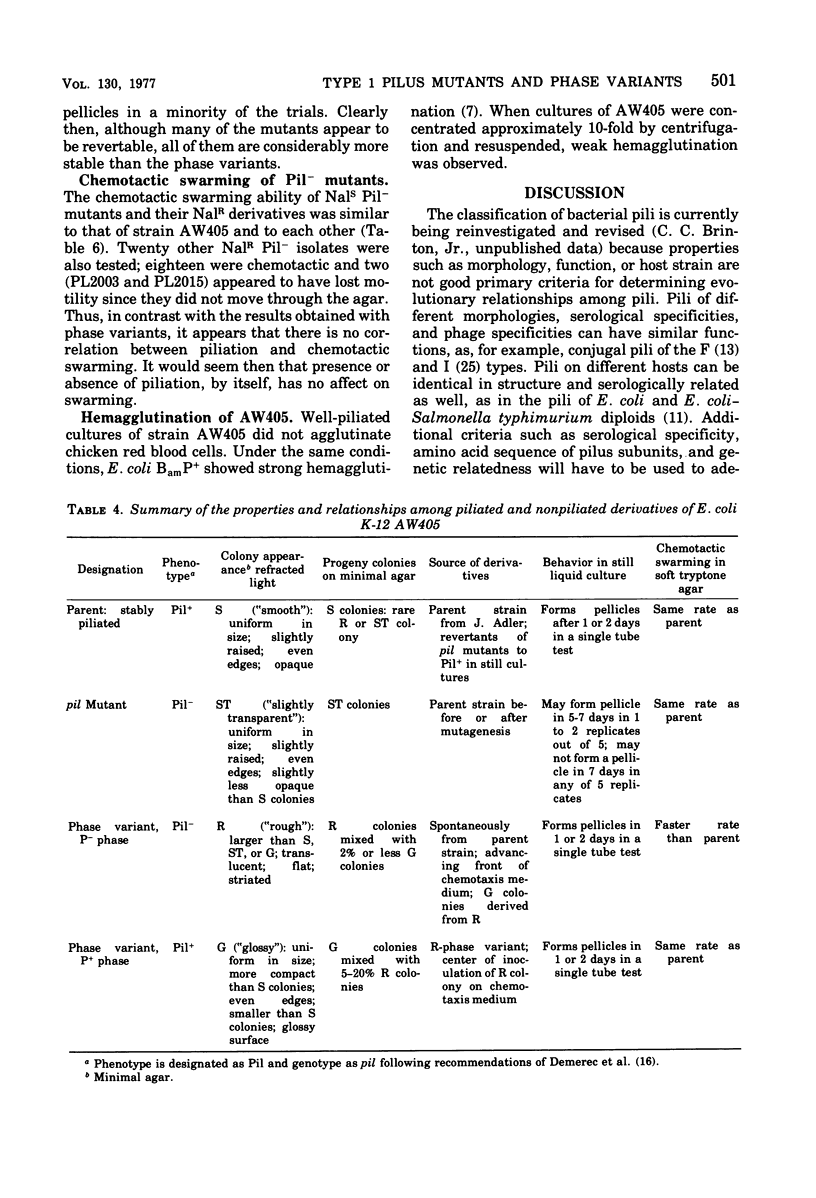
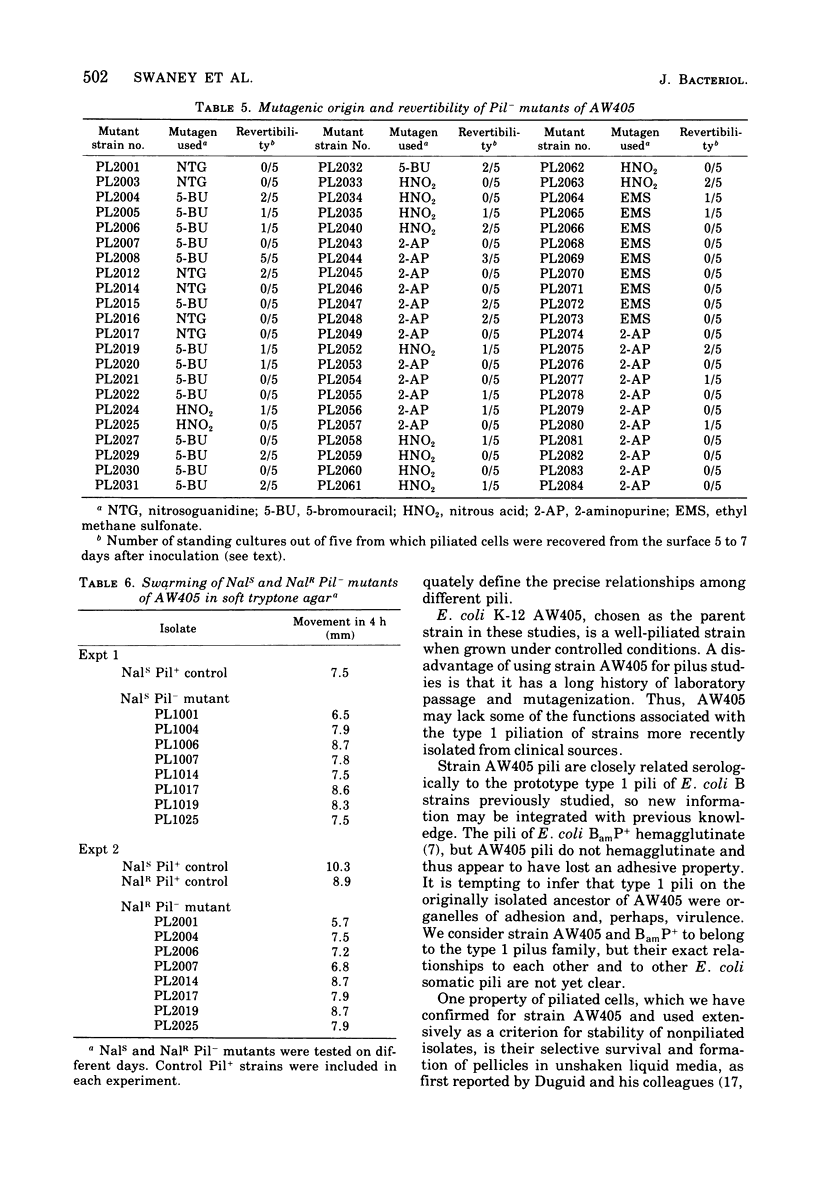



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, BUZZELL A., LAUFFER M. A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954 Dec;15(4):533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINTON C. C., Jr Non-flagellar appendages of bacteria. Nature. 1959 Mar 21;183(4664):782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovre K., Froholm L. O. Competence of genetic transformation correlated with the occurrence of fimbriae in three bacterial species. Nat New Biol. 1971 Dec 1;234(48):151–152. [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froholm L. O., Bovre K. Fimbriation associated with the spreading-corroding colony type in Moraxella kingii. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):641–648. [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen J., Froholm L. O., Bovre K. Studies on bacterial surface translocation. 2. Correlation of twitching motility and fimbriation in colony variants of Moraxella nonliquefaciens, M. bovis, and M. kingii. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(3):445–452. [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Sex pili and common pili in the conjugational transfer of colicin factor Ib by Salmonella typhimurium. Genet Res. 1967 Jun;9(3):359–367. doi: 10.1017/s0016672300010636. [DOI] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., Ippen-Ihler K., Brinton C. C., Jr Genetic complementation analysis of Escherichia coli type 1 somatic pilus mutants. J Bacteriol. 1977 Apr;130(1):506–511. doi: 10.1128/jb.130.1.506-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weppelman R. M., Brinton C. C., Jr The infection of Pseudomonas aeruginosa by RNA pilus phage PP7: the adsorption organelle and the relationship between phage sensitivity and the division cycle. Virology. 1971 Apr;44(1):1–17. doi: 10.1016/0042-6822(71)90147-4. [DOI] [PubMed] [Google Scholar]