Abstract
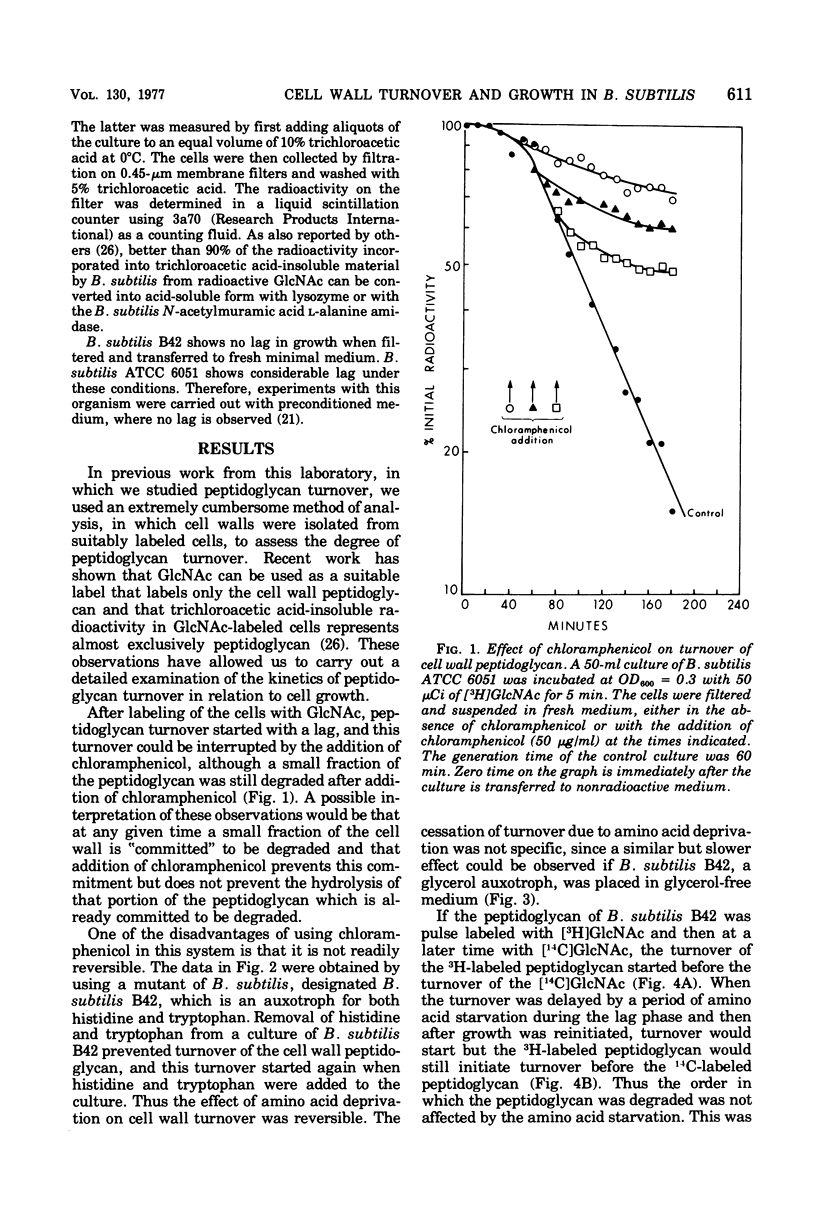
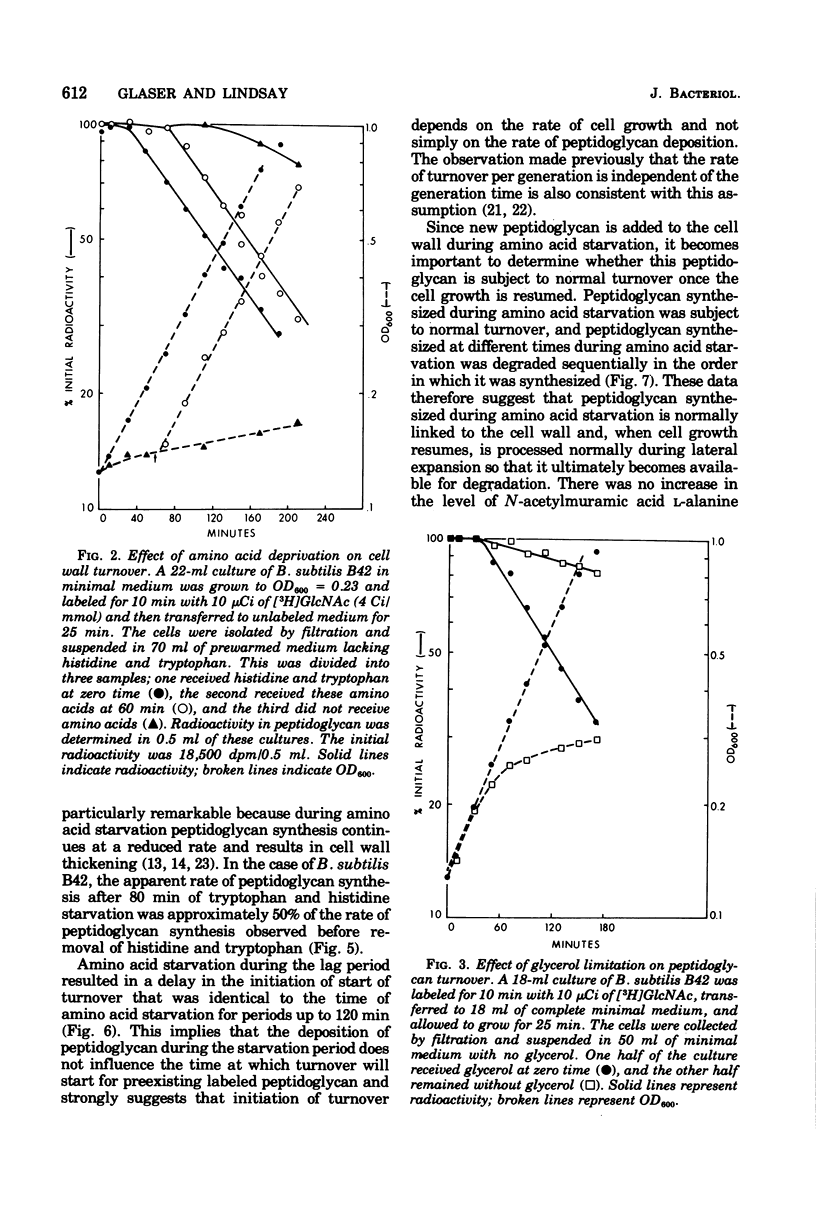
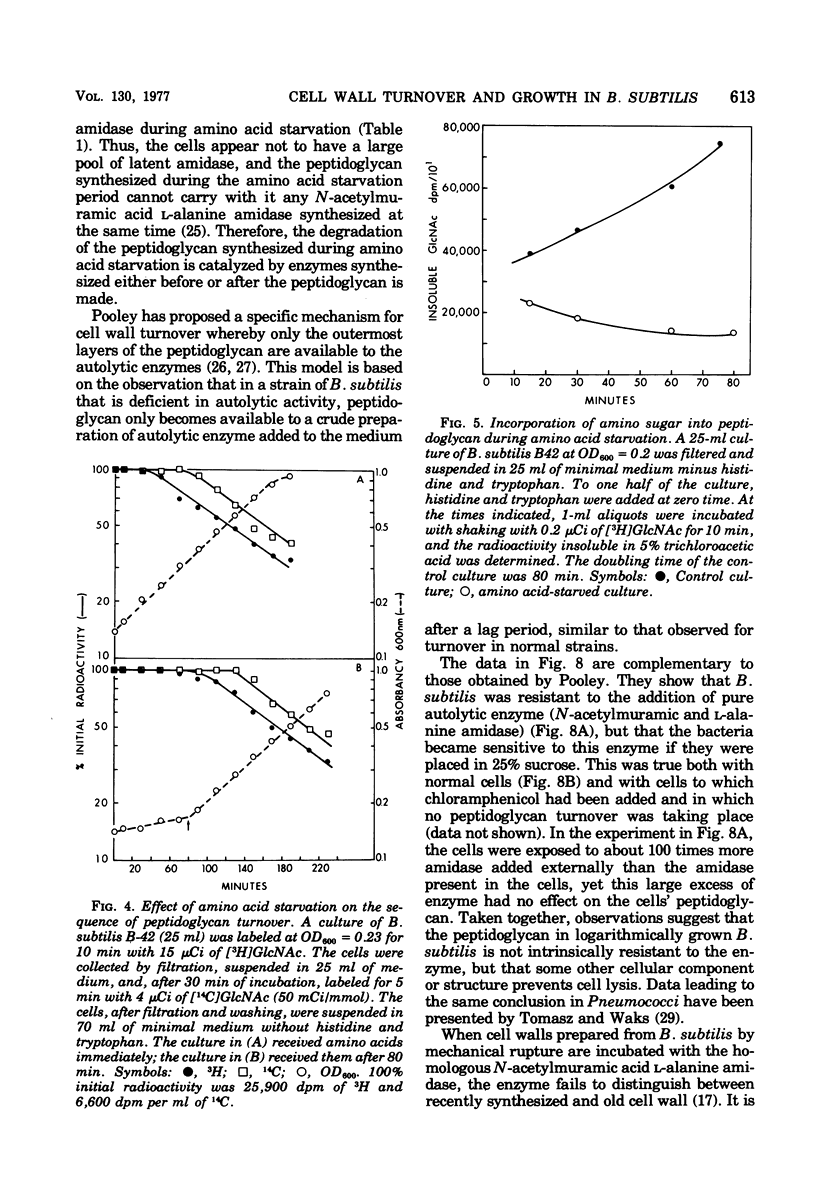
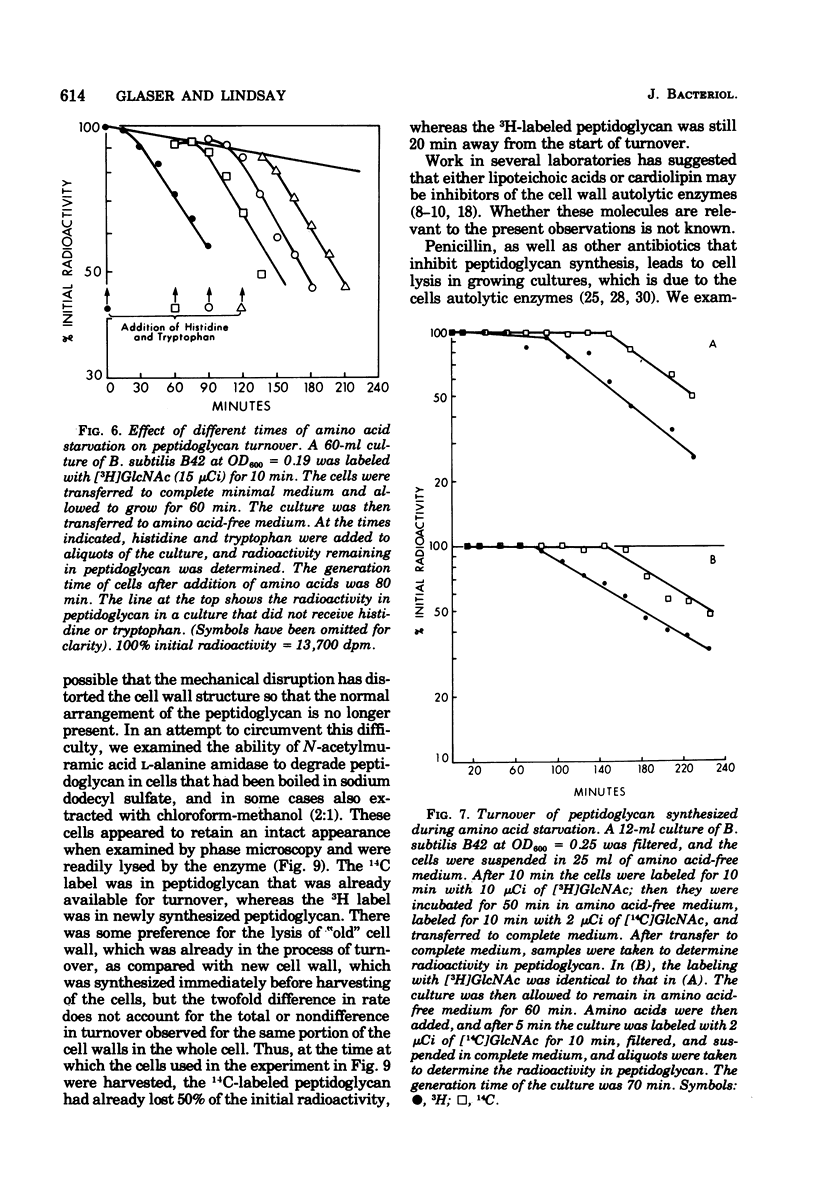
The kinetics of cell wall turnover in Bacillus subtilis have been examined in detail. After pulse labeling of the peptidoglycan with N-acetylglucosamine, the newly formed peptidoglycan is stable for approximately three-quarters of a generation and is then degraded by a process that follows first-order kinetics. Deprivation of an auxotroph of amino acids required for protein synthesis results in a cessation of turnover. If a period of amino acid starvation occurs during the lag phase of turnover, then the initiation of turnover is delayed for a period of time equivalent to the starvation period. During amino acid starvation, new cell wall peptidoglycan is synthesized and added to preexisting cell wall. This peptidoglycan after resumption of growth is also subject to degradation (turnover). It is suggested that cell wall turnover is dependent on cell growth and elongation. Several possible control mechanisms for cell wall autolytic enzymes are discussed in light of these observations.
Full text
PDF

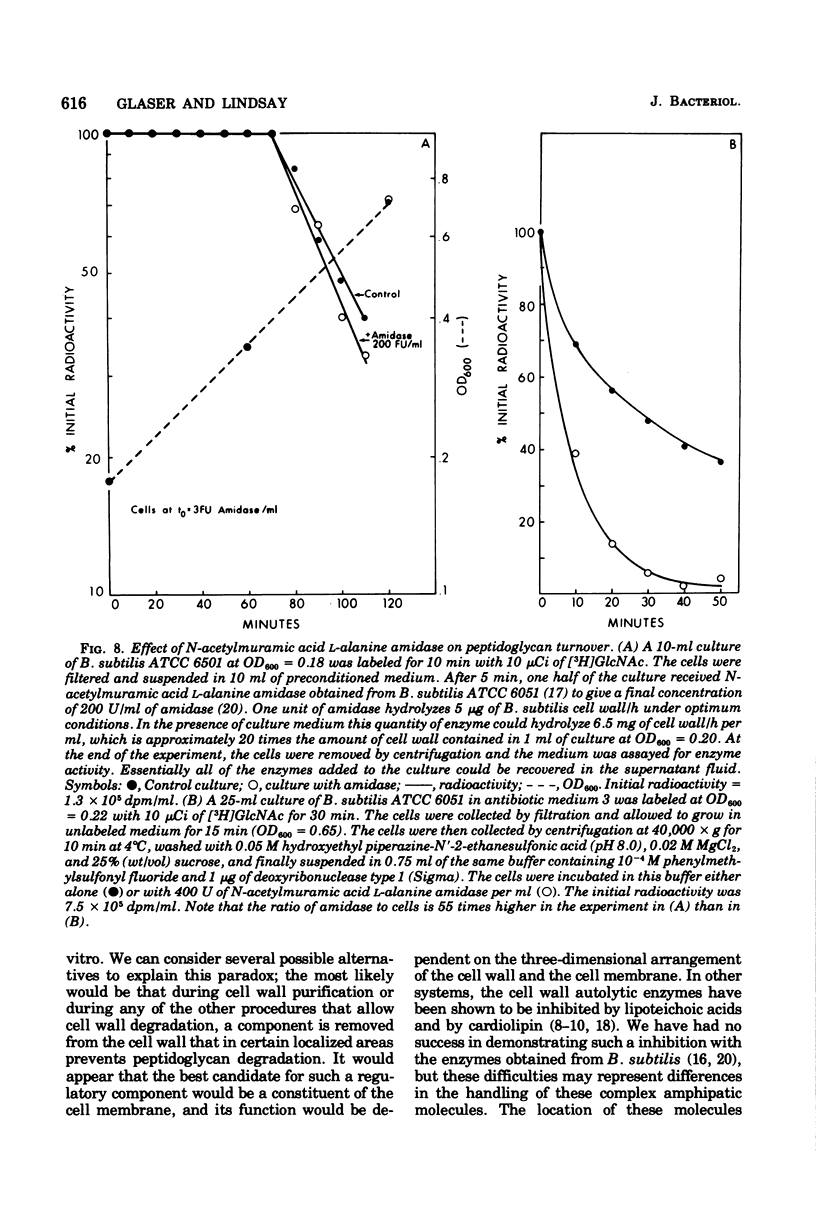
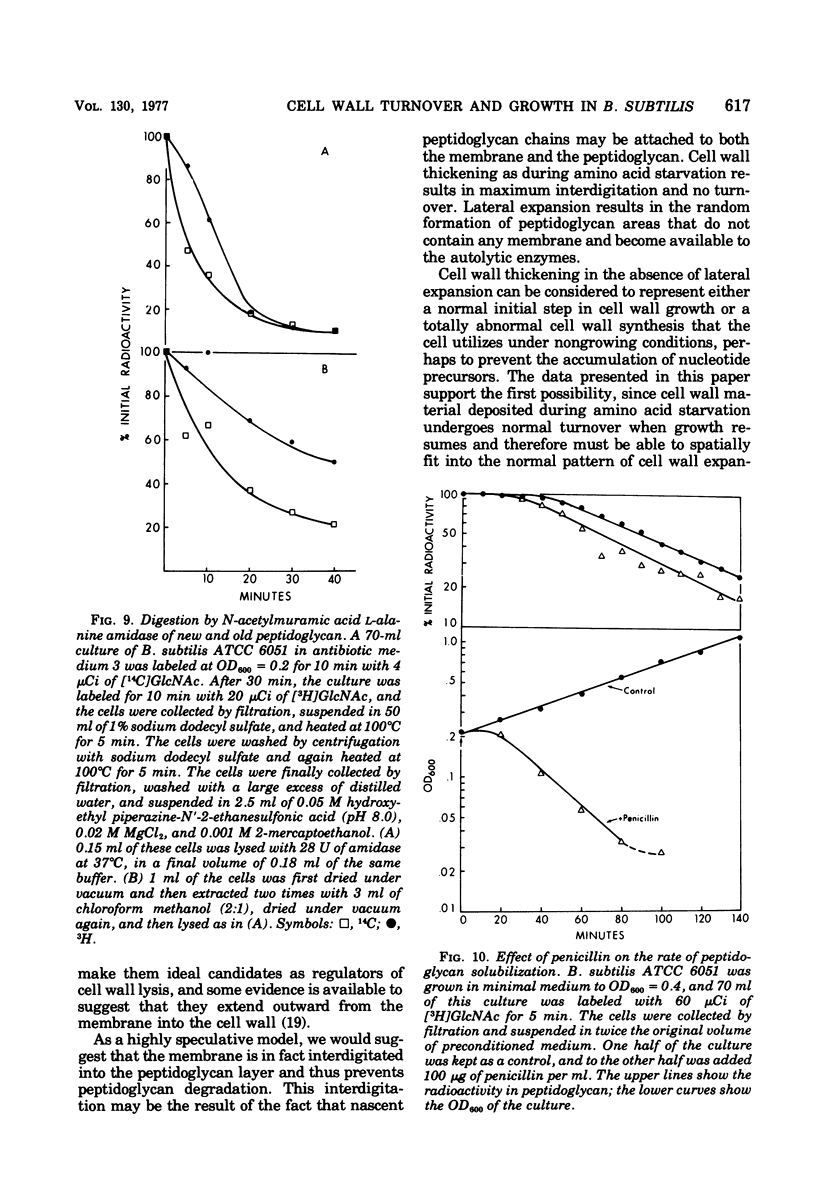
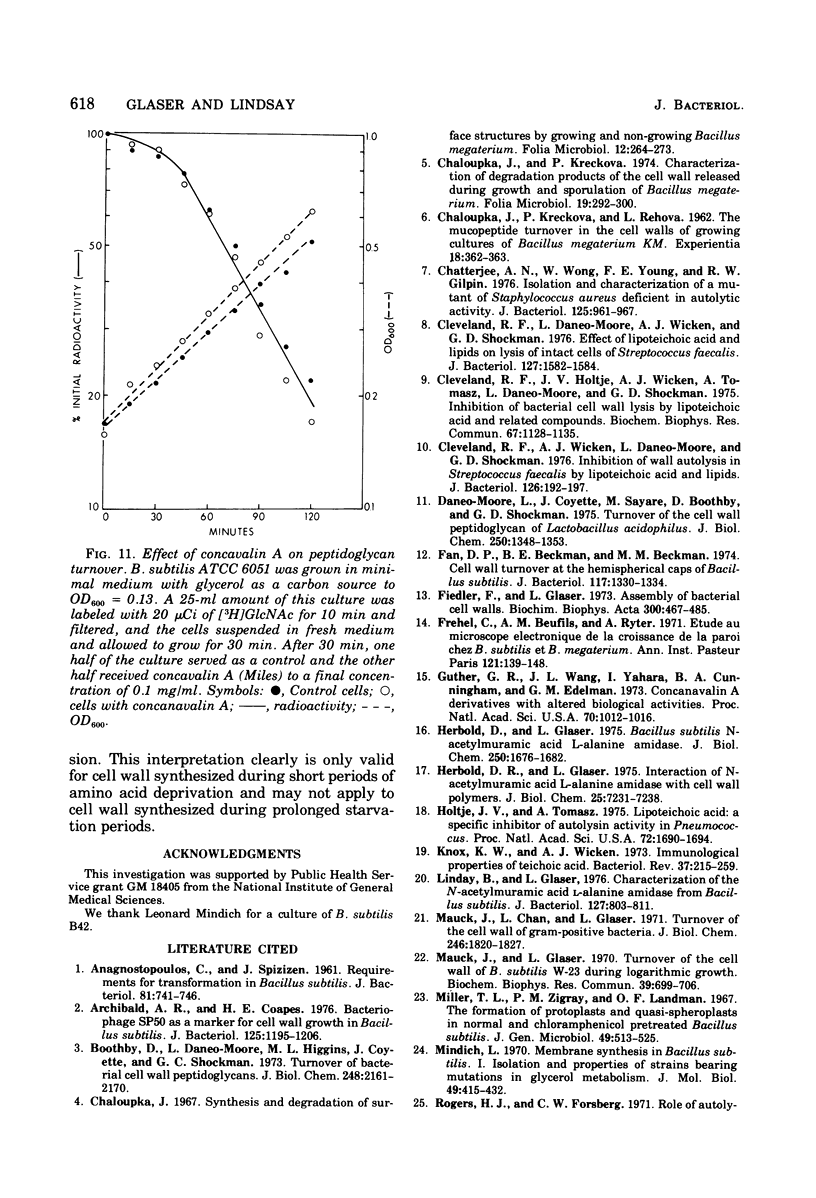

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E. Bacteriophage SP50 as a marker for cell wall growth in Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1195–1206. doi: 10.1128/jb.125.3.1195-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- CHALOUPKA J., KRECKOVA P., RIHOVA L. The mucopeptide turnover in the cell walls of growing cultures of Bacillus megaterium KM. Experientia. 1962 Aug 15;18:362–363. doi: 10.1007/BF02172250. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Characterization of degradation products of the cell wall released during growth and sporulation of Bacillus megaterium. Folia Microbiol (Praha) 1974;19(4):292–300. doi: 10.1007/BF02873221. [DOI] [PubMed] [Google Scholar]
- Chaloupka J. Synthesis and degradation of surface structures by growing and non-growing Bacillus megaterium. Folia Microbiol (Praha) 1967;12(3):264–273. doi: 10.1007/BF02868742. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Beckman M. M. Cell wall turnover at the hemispherical caps of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1330–1334. doi: 10.1128/jb.117.3.1330-1334.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder F., Glaser L. Assembly of bacterial cell walls. Biochim Biophys Acta. 1973 Dec 28;300(4):467–485. doi: 10.1016/0304-4157(73)90016-6. [DOI] [PubMed] [Google Scholar]
- Frehel C., Beaufils A. M., Ryter A. Etude au microscope électronique de la croissance de la paroi chez B. subtilis et B. megaterium. Ann Inst Pasteur (Paris) 1971 Aug;121(2):139–148. [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay B., Glaser L. Characterization of the N-acetylmuramic acid L-alanine amidase from Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):803–811. doi: 10.1128/jb.127.2.803-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem Biophys Res Commun. 1970 May 22;39(4):699–706. doi: 10.1016/0006-291x(70)90261-5. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Enzyme replacement in a bacterium: phenotypic correction by the experimental introduction of the wild type enzyme into a live enzyme defective mutant pneumococcus. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1311–1319. doi: 10.1016/s0006-291x(75)80373-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


