Abstract
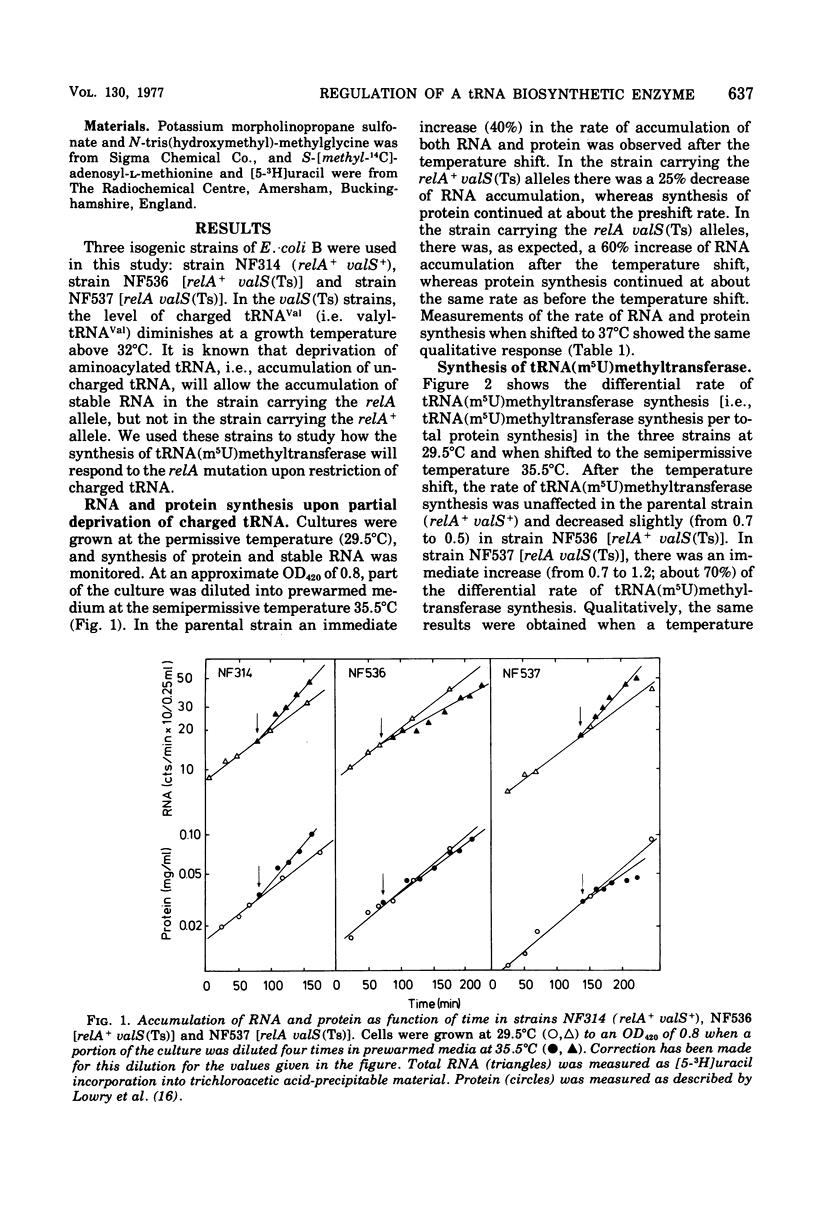
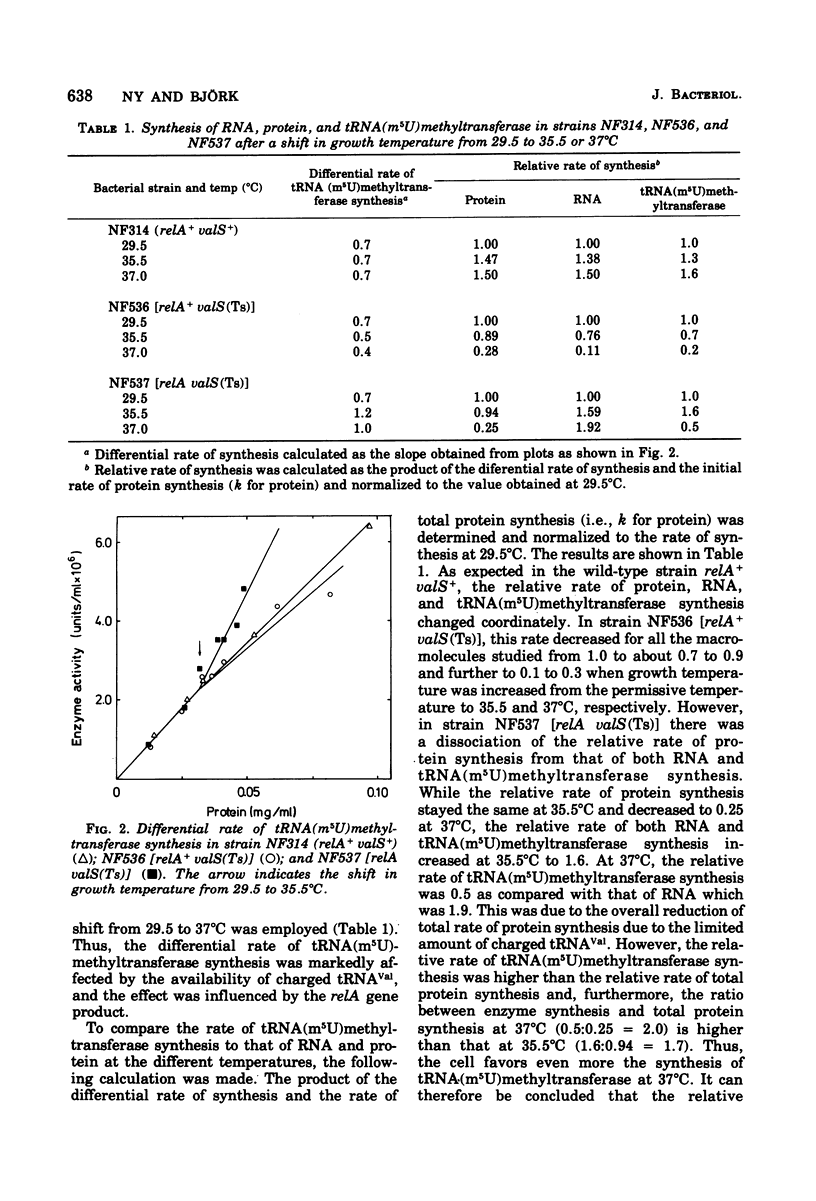
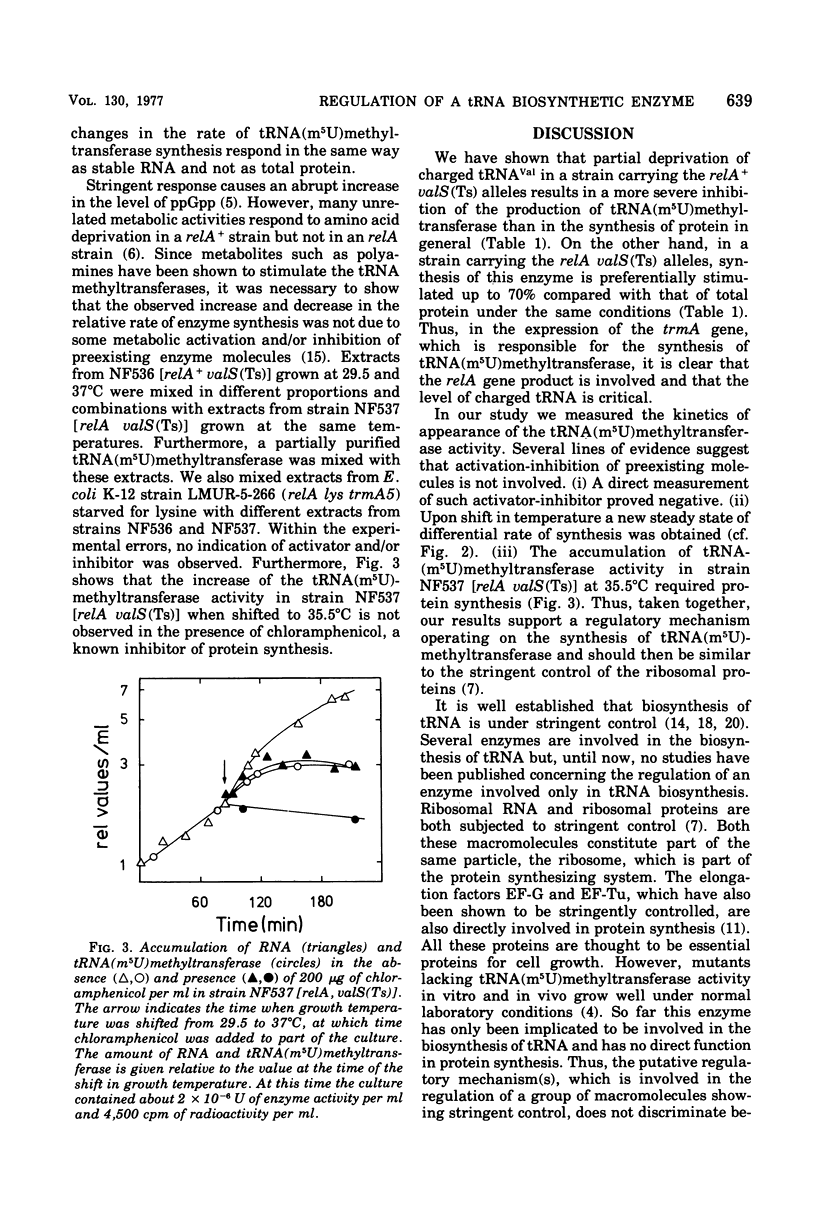
This paper describes the regulation of a transfer ribonucleic acid (tRNA) biosynthetic enzyme, the tRNA(m5U)methyltransferase (EC 2.1.1.35). This enzyme catalyzes the formation of 5-methyluridine (m5U, ribothymidine) in all tRNA chains of Escherichia coli. Partial deprivation of charged tRNAVal can be imposed by shifting strains carrying a temperature-sensitive valyl-tRNA ligase from a permissive to a semipermissive temperature. By using two such strains differing only in the allelic state of the relA gene, it was possible to show the tRNA(m5U)methyltransferase to be stringently regulated. Upon partial deprivation of charged tRNAVal, the differential rate of tRNA(m5U)methyltransferase synthesis was found to decrease in a strain with stringent RNA control (relA+), whereas it increased in the strain carrying the relA allele. This increase of accumulation of tRNA(m5U)methyltransferase activity required protein synthesis. Thus, when tRNA is partially uncharged in the cell, the relA gene product influences the expression of tRNA(m5U)methyltransferase gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- Björk G. R. Identification of bacteriophage T4-specific precursor tRNA by using a host mutant defective in the methylation of tRNA. J Virol. 1975 Sep;16(3):741–744. doi: 10.1128/jvi.16.3.741-744.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of the transcriptional activities of ribosomal protein genes in E. coli. Nature. 1975 Jun 5;255(5508):460–465. doi: 10.1038/255460a0. [DOI] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Furano A. V., Wittel F. P. Effect of the RelA gene on the synthesis of individual proteins in vivo. Cell. 1976 May;8(1):115–122. doi: 10.1016/0092-8674(76)90192-6. [DOI] [PubMed] [Google Scholar]
- Furano A. V., Wittel F. P. Syntheses of elongation factors Tu and G are under stringent control in Escherichia coli. J Biol Chem. 1976 Feb 10;251(3):898–901. [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. II. Noncoordinate accumulation during stringent control. J Biol Chem. 1973 Jul 25;248(14):5033–5041. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leboy P. S. Stimulation of soluble ribonucleic acid methylase activity by polyamines. Biochemistry. 1970 Mar 31;9(7):1577–1584. doi: 10.1021/bi00809a016. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., EIDLIC L. Characterization of the RNA formed under conditions of relaxed amino acid control in Escherichia coli. Biochim Biophys Acta. 1963 Mar 26;68:380–388. doi: 10.1016/0006-3002(63)90159-8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Ryan A. M., Borek E. The relaxed control phenomenon. Prog Nucleic Acid Res Mol Biol. 1971;11:193–228. doi: 10.1016/s0079-6603(08)60328-1. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Shimura Y., Ozeki H. Selective modification of nucleosides of tRNA precursors accumulated in a temperature sensitive mutant of Escherichia coli. FEBS Lett. 1974 Nov 1;48(1):117–121. doi: 10.1016/0014-5793(74)81076-8. [DOI] [PubMed] [Google Scholar]
- Schaefer K. P., Altman S., Söll D. Nucleotide modification in vitro of the precursor of transfer RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3626–3630. doi: 10.1073/pnas.70.12.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D. Transcription and processing of transfer RNA precursors. Prog Nucleic Acid Res Mol Biol. 1976;16:25–73. doi: 10.1016/s0079-6603(08)60755-2. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMIR A., HOLLEY R. W., MARQUISEE M. EVIDENCE FOR THE OCCURRENCE OF A COMMON PENTANUCLEOTIDE SEQUENCE IN THE STRUCTURES OF TRANSFER RIBONUCLEIC ACIDS. J Biol Chem. 1965 Mar;240:1267–1273. [PubMed] [Google Scholar]


