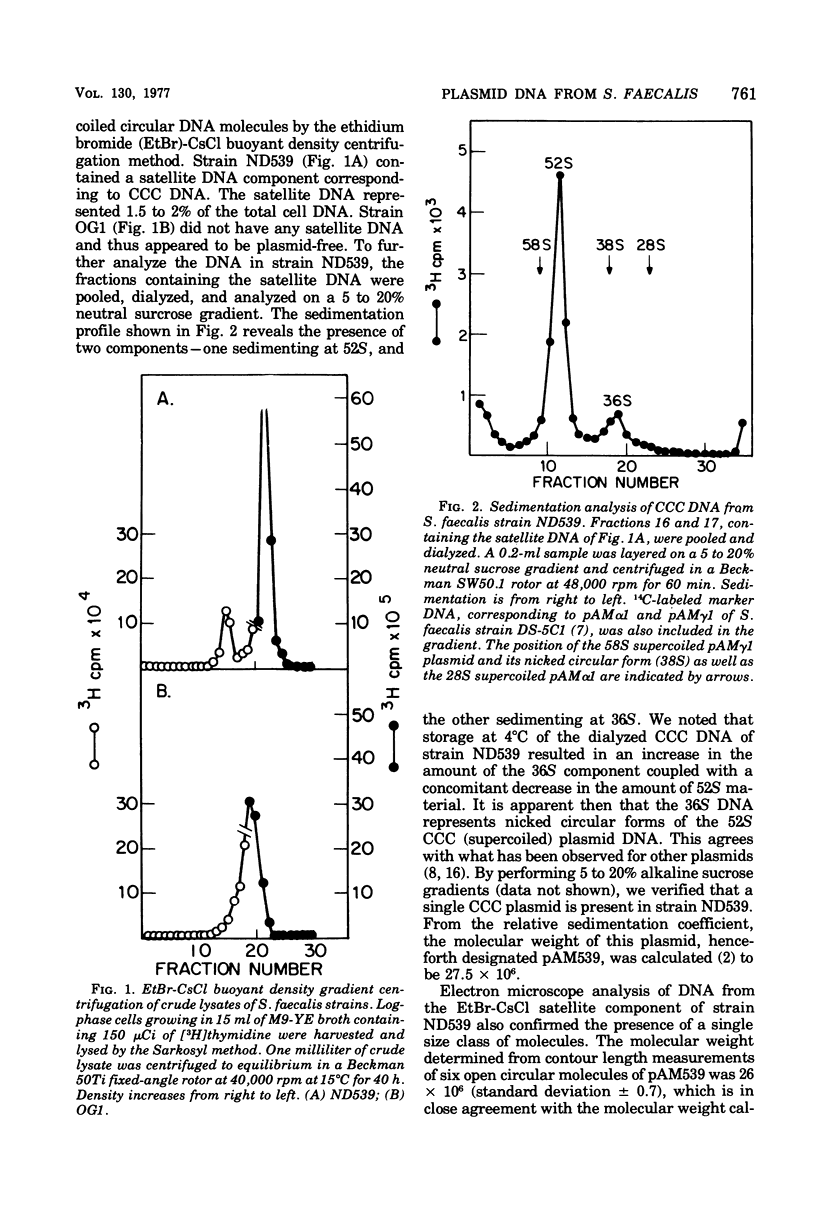
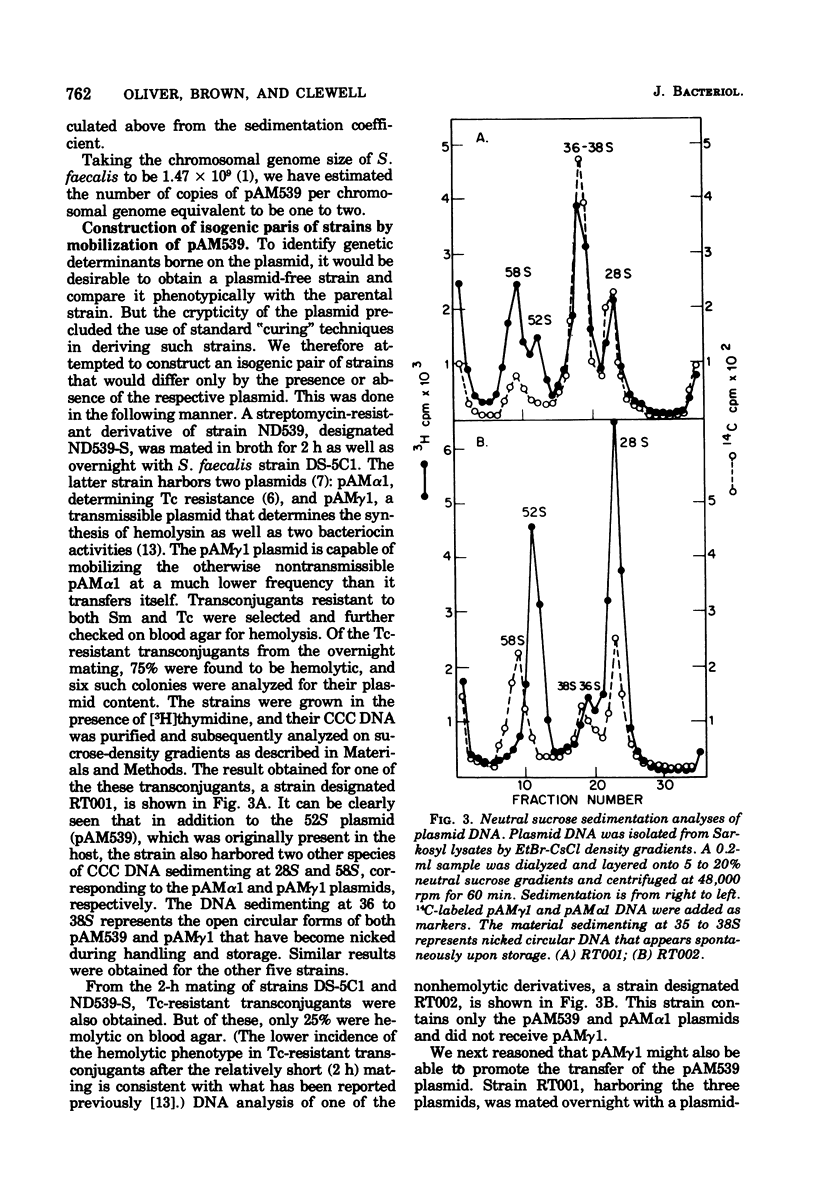
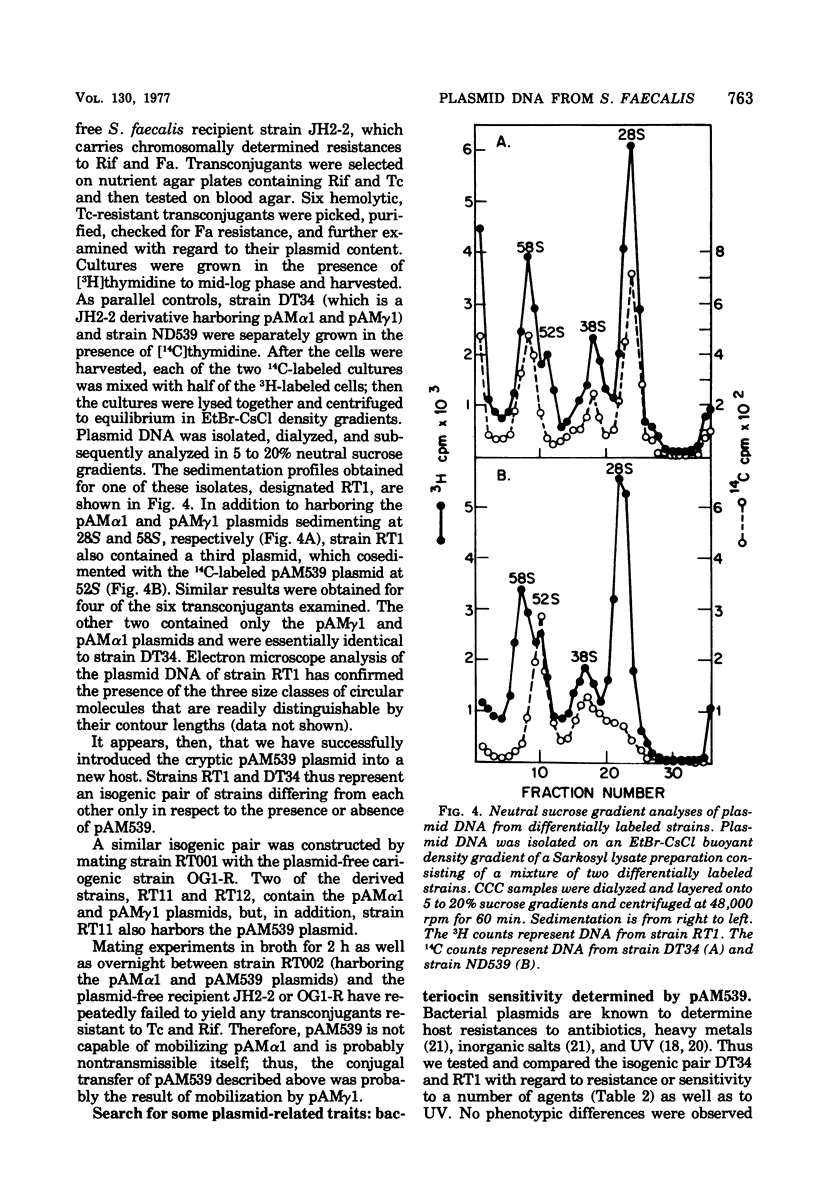
Abstract
Streptococcus faecalis strains ND539 and OG1 have been previously shown to be cariogenic in gnotobiotic animals. Deoxyribonucleic acid analyses have revealed the presence of a single 26-megadalton plasmid designated pAM539 in the former strain, whereas the latter strain was found to be plasmid-free. By gene transfer experiments, it was possible to construct isogenic pairs of strains that differed only with regard to the presence or absence of pAM539. Comparative studies of isogenic pairs showed that the presence of pAM539 conferred bacterial sensitivity to a bacteriocin produced by S. faecalis strain 5952.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. HYDROLYSIS OF PROTEINS AND NUCLEIC ACIDS BY LANCEFIELD GROUP A AND OTHER STREPTOCOCCI. J Bacteriol. 1963 Dec;86:1270–1274. doi: 10.1128/jb.86.6.1270-1274.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Araya S., Higuchi M. Plasmid DNA satellite bands seen in lysates of Streptococcus mutans that form insoluble extracellular polysaccharides. J Dent Res. 1976 Mar-Apr;55(2):266–271. doi: 10.1177/00220345760550021801. [DOI] [PubMed] [Google Scholar]
- Howarth S. Resistance to the bactericidal effect of ultraviolet radiation conferred on Enterobacteria by the colicine factor coli. J Gen Microbiol. 1965 Jul;40(1):43–55. doi: 10.1099/00221287-40-1-43. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G. Effects of an R factor and caffeine on ultraviolet mutability in Salmonella typhimurium. Mutat Res. 1973 Jun;18(3):367–370. doi: 10.1016/0027-5107(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., ERVIN R. F., GORDON H. A., WAGNER M. Experimental caries in germfree rats inoculated with enterococci. J Am Dent Assoc. 1955 Mar;50(3):259–272. doi: 10.14219/jada.archive.1955.0061. [DOI] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS N. B., FORBES M. A., BLAU E., EICKENBERG C. F. A study of the simultaneous occurrence of Enterococci, Lactobacilli, and yeasts in saliva from human beings. J Dent Res. 1950 Oct;29(5):563–570. doi: 10.1177/00220345500290050201. [DOI] [PubMed] [Google Scholar]
- WINKLER K. C., VAN AMERONGEN J. Bacteriologic results from 4,000 root canal cultures. Oral Surg Oral Med Oral Pathol. 1959 Jul;12(7):857–875. doi: 10.1016/0030-4220(59)90036-2. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


