Abstract
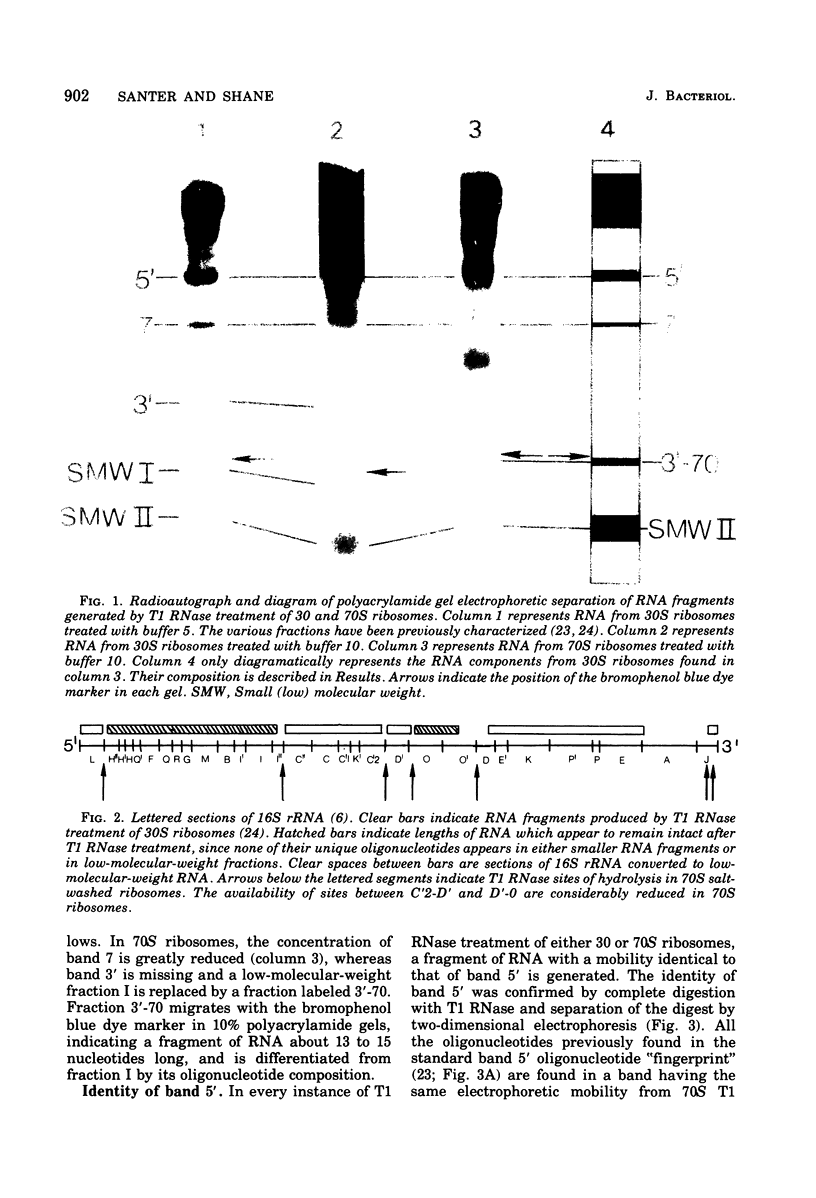
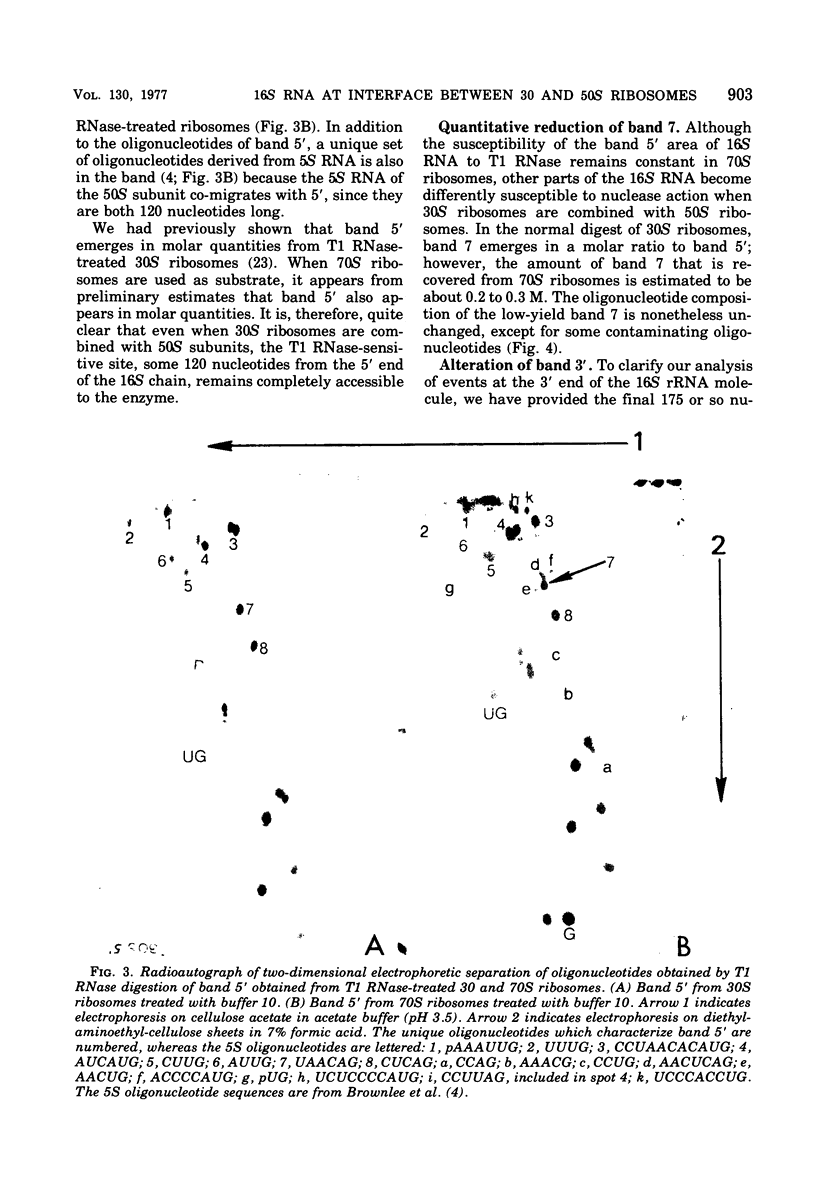
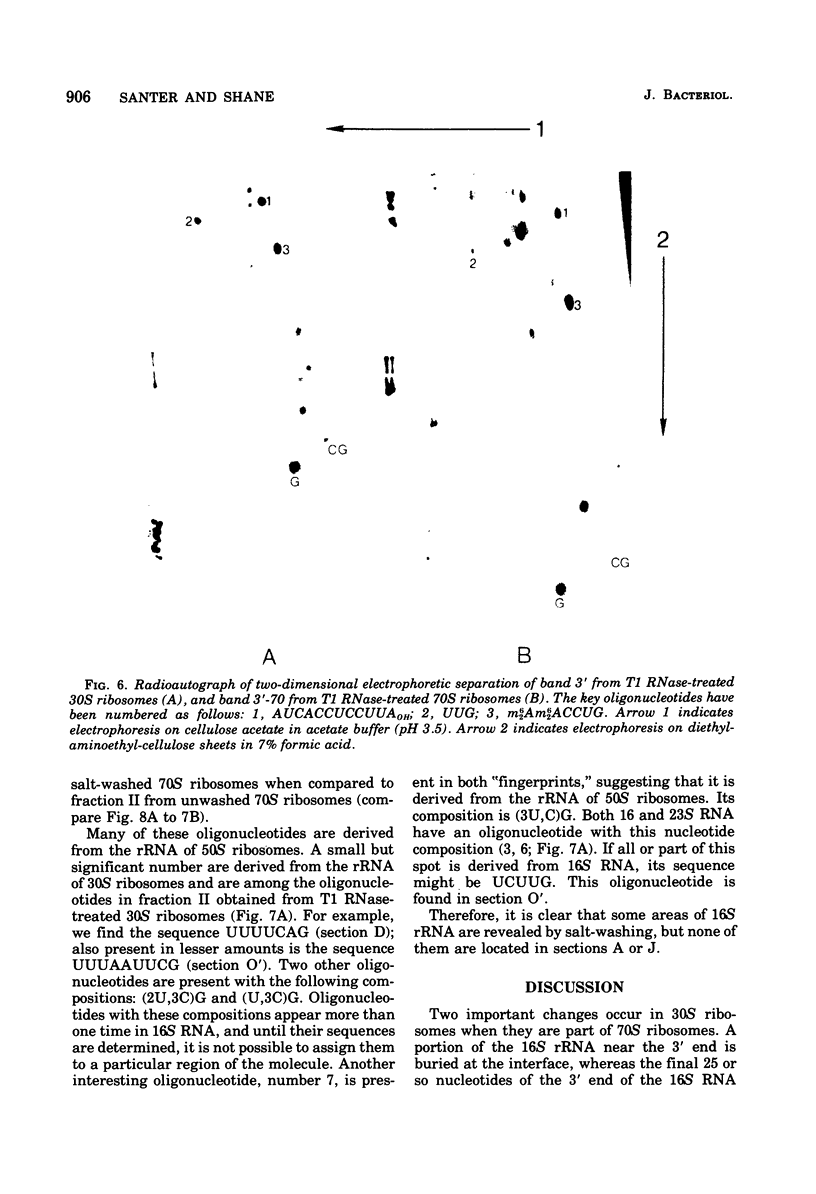
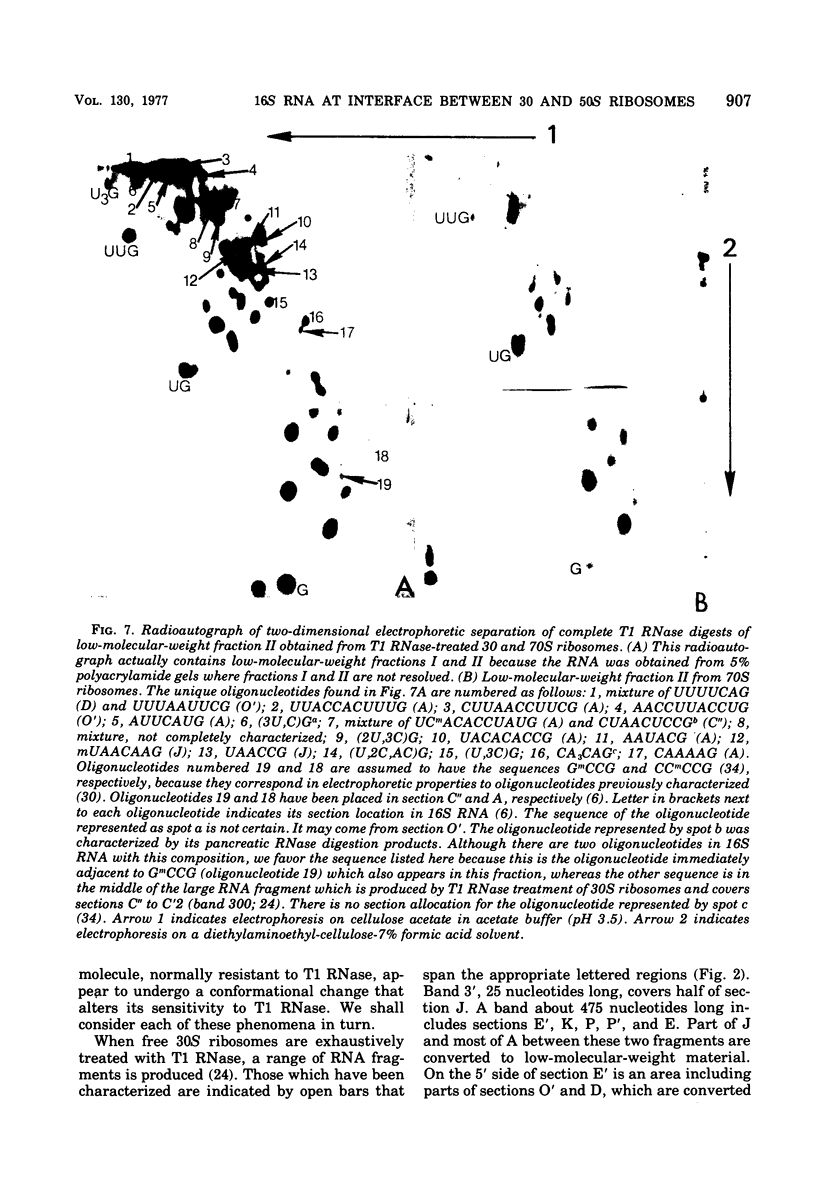
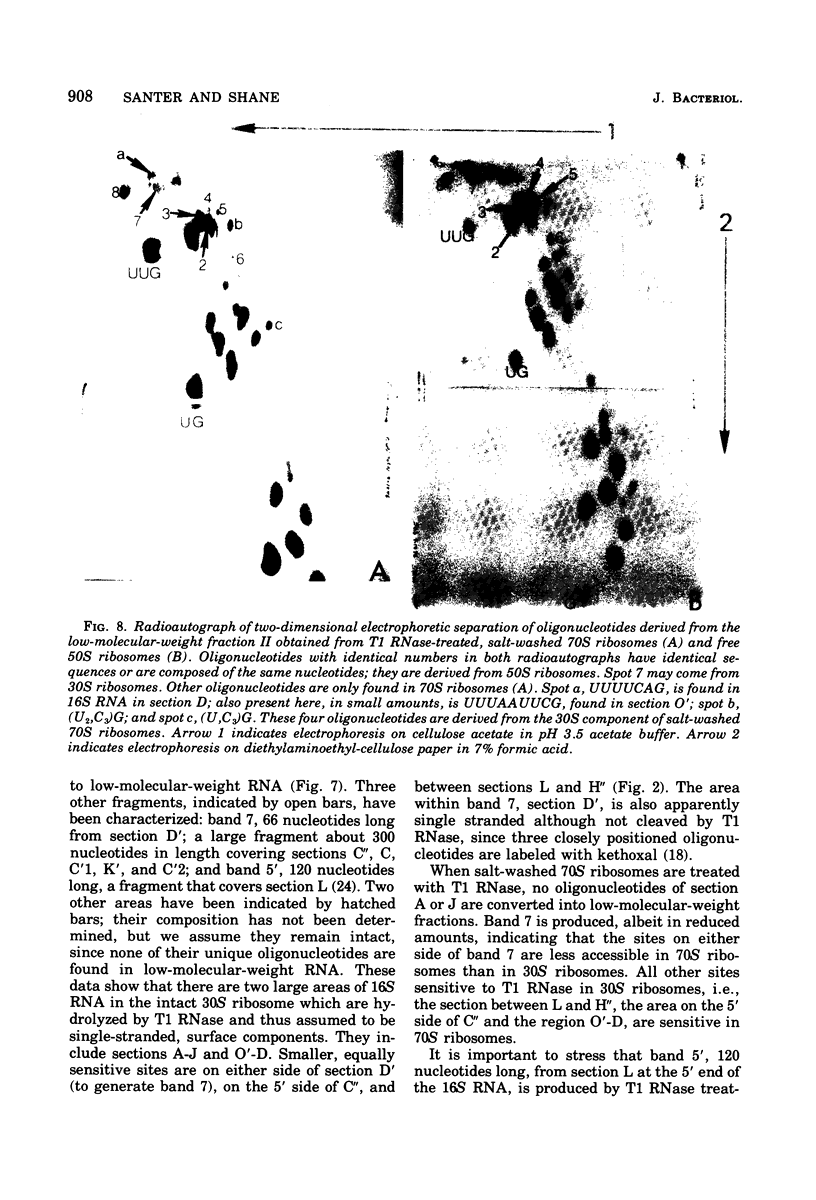
To determine the region of 16S ribonucleic acid (RNA) at the interface between 30 and 50S ribosomes of Escherichia coli, 30 and 70S ribosomes were treated with T1 ribonuclease (RNase). The accessibility of 16S RNA in the 5' half of the molecule is the same in 30 and 70S ribosomes. The interaction with 50S ribosomes decreases the sensitivity to T1 RNase of an area in the middle of 16S RNA. A large area near the 3' end of 16S RNA is completely protected in 70S ribosomes. The RNA near the 3' end of the molecule and an area of RNA in the middle of the molecule appear to be at the interface between 30 and 50S ribosomes. One site in 16S RNA, 13 to 15 nucleotides from the 3' end, normally inaccessible to T1 RNase in 30S ribosomes, becomes accessible to T1 RNase in 70S ribosomes. This indicates a conformational change at the 3' end of 16S RNA when 30S ribosomes are associated with 50S ribosomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Jeppesen P. G., Sanger F., Barrell B. G. Nucleotide sequence from the coat protein cistron of R17 bacteriophage RNA. Nature. 1969 Sep 6;223(5210):1009–1014. doi: 10.1038/2231009a0. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Widada J. S., Krol A., Fellner P., Ebel J. P. Nucleotide sequences of the T1 and pancreatic ribonuclease digestion products from some large fragments of the 23S RNA of Escherichia coli. Biochimie. 1975;57(2):175–225. doi: 10.1016/s0300-9084(75)80168-4. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Chang F. N. Letter: Conformational changes in ribosomal subunits following dissociation of the Escherichia coli 70 S ribosome. J Mol Biol. 1973 Aug 15;78(3):563–568. doi: 10.1016/0022-2836(73)90476-2. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Reynier M. Resistance of E. coli ribosomal 5S RNA to degradation by ribonuclease in reconstituted ribosomal particles. Biochem Biophys Res Commun. 1970 Apr 8;39(1):114–122. doi: 10.1016/0006-291x(70)90765-5. [DOI] [PubMed] [Google Scholar]
- Hawley D. A., Slobin L. I. The immunological reactivity of 30s ribosomal proteins in 70s ribosomes from Escherichia coli. Biochem Biophys Res Commun. 1973 Nov 1;55(1):162–168. doi: 10.1016/s0006-291x(73)80073-7. [DOI] [PubMed] [Google Scholar]
- Highland J. H., Ochsner E., Gordon J., Bodley J. W., Hasenbank R., Stöffler G. Coordinate inhibition of elongation factor G function and ribosomal subunit association by antibodies to several ribosomal proteins. Proc Natl Acad Sci U S A. 1974 Mar;71(3):627–630. doi: 10.1073/pnas.71.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. H., Cantor C. R. Studies of 30 S Escherichia coli ribosome reassembly using individual proteins labeled with an environmentally sensitive fluorescent prode. J Mol Biol. 1975 Oct 5;97(4):423–441. doi: 10.1016/s0022-2836(75)80052-0. [DOI] [PubMed] [Google Scholar]
- Huang K., Cantor C. R. Surface topography of the 30 s Escherichia coli ribosomal subunit: reactivity towards fluorescein isothiocyanate. J Mol Biol. 1972 Jun 20;67(2):265–275. doi: 10.1016/0022-2836(72)90240-9. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Zeichhardt H., Kurland C. G. Ribosomal protein neighborhoods. I. S18 and S21 as well as S5 and S8 are neighbors. Mol Gen Genet. 1972;119(4):357–366. [PubMed] [Google Scholar]
- Mackie G. A., Zimmermann R. A. Characterization of fragments of 16 S ribonucleic acid protected against pancreatic ribonuclease digestion by ribosomal protein S4. J Biol Chem. 1975 Jun 10;250(11):4100–4112. [PubMed] [Google Scholar]
- Miller R. V., Sypherd P. S. Topography of the Escherichia coli 30 S ribosome revealed by the modification of ribosomal proteins. J Mol Biol. 1973 Aug 15;78(3):539–550. doi: 10.1016/0022-2836(73)90474-9. [DOI] [PubMed] [Google Scholar]
- Morrison C. A., Garrett R. A., Zeichhardt H., Stöffler G. Proteins occurring at, or near, the subunit interface of E. coli ribosomes. Mol Gen Genet. 1973 Dec 31;127(4):359–368. doi: 10.1007/BF00267106. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Nucleotide sequence of the 3' terminus of E. coli 16S ribosomal RNA. Mol Biol Rep. 1974 Dec;1(8):437–439. doi: 10.1007/BF00360668. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Topography of 16S RNA in 30S ribosomal subunits. Nucleotide sequences and location of sites of reaction with kethoxal. Biochemistry. 1974 Nov 5;13(23):4694–4703. doi: 10.1021/bi00720a003. [DOI] [PubMed] [Google Scholar]
- Pon C. L., Gualerzi C. The role of 16S rRNA in ribosomal binding of IF-3. Biochemistry. 1976 Feb 24;15(4):804–811. doi: 10.1021/bi00649a012. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Santer M., Santer U. V. Characterization of the 5' and 3' ends of the 16S ribonucleic acid from T 1 -ribonuclease treated 30S ribosomes. Biochemistry. 1972 Feb 29;11(5):786–791. doi: 10.1021/bi00755a017. [DOI] [PubMed] [Google Scholar]
- Santer M., Santer U. Action of ribonuclease T1 on 30S ribosomes of Escherichia coli and its role in sequence studies on 16S ribonucleic acid. J Bacteriol. 1973 Dec;116(3):1304–1313. doi: 10.1128/jb.116.3.1304-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Traut R. R., Kahan L. Protein-protein proximity in the association of ribosomal subunits of Escherichia coli: crosslinking of 30 S protein S16 to 50 S proteins by glutaraldehyde or formaldehyde. J Mol Biol. 1974 Aug 15;87(3):509–522. doi: 10.1016/0022-2836(74)90101-6. [DOI] [PubMed] [Google Scholar]
- Uchida T., Bonen L., Schaup H. W., Lewis B. J., Zablen L., Woese C. The use of ribonuclease U2 in RNA sequence determination. Some corrections in the catalog of oligomers produced by ribonuclease T1 digestion of Escherichia coli 16S ribosomal RNA. J Mol Evol. 1974 Feb 28;3(1):63–77. doi: 10.1007/BF01795977. [DOI] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]