Abstract
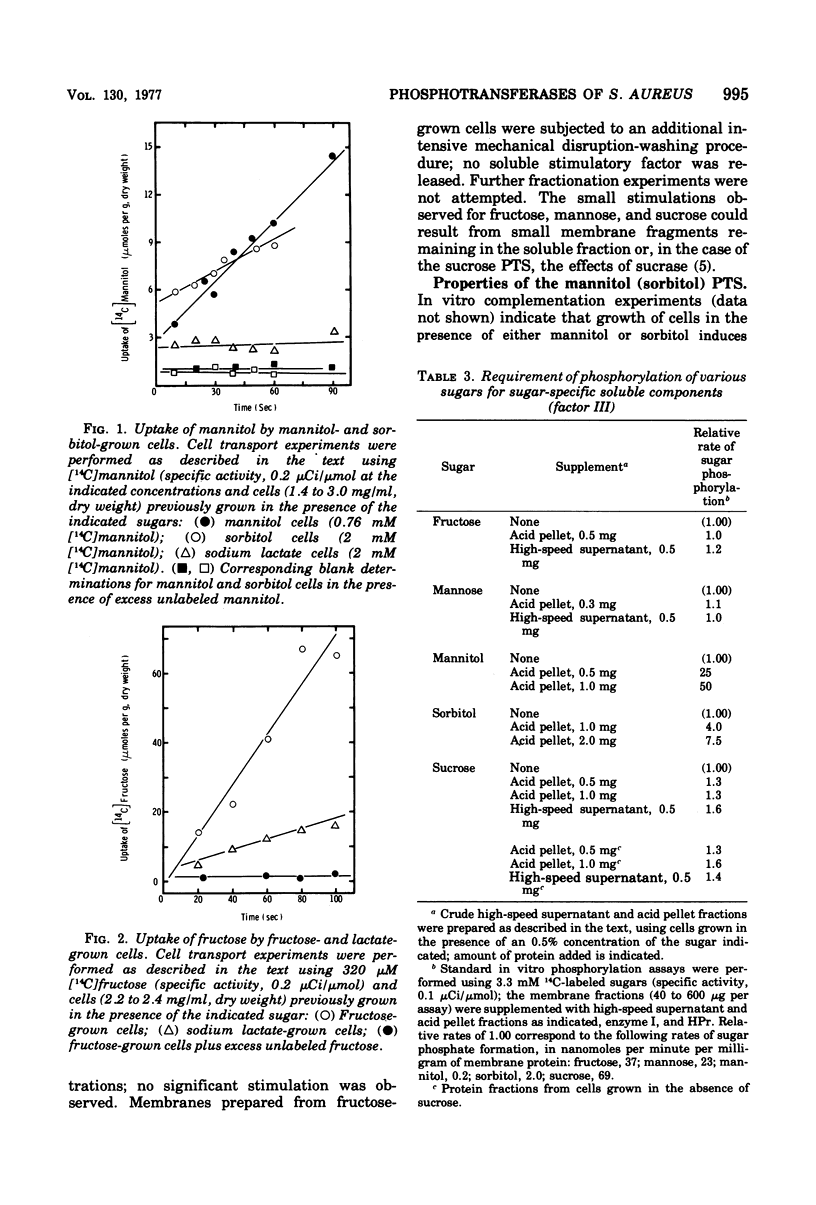
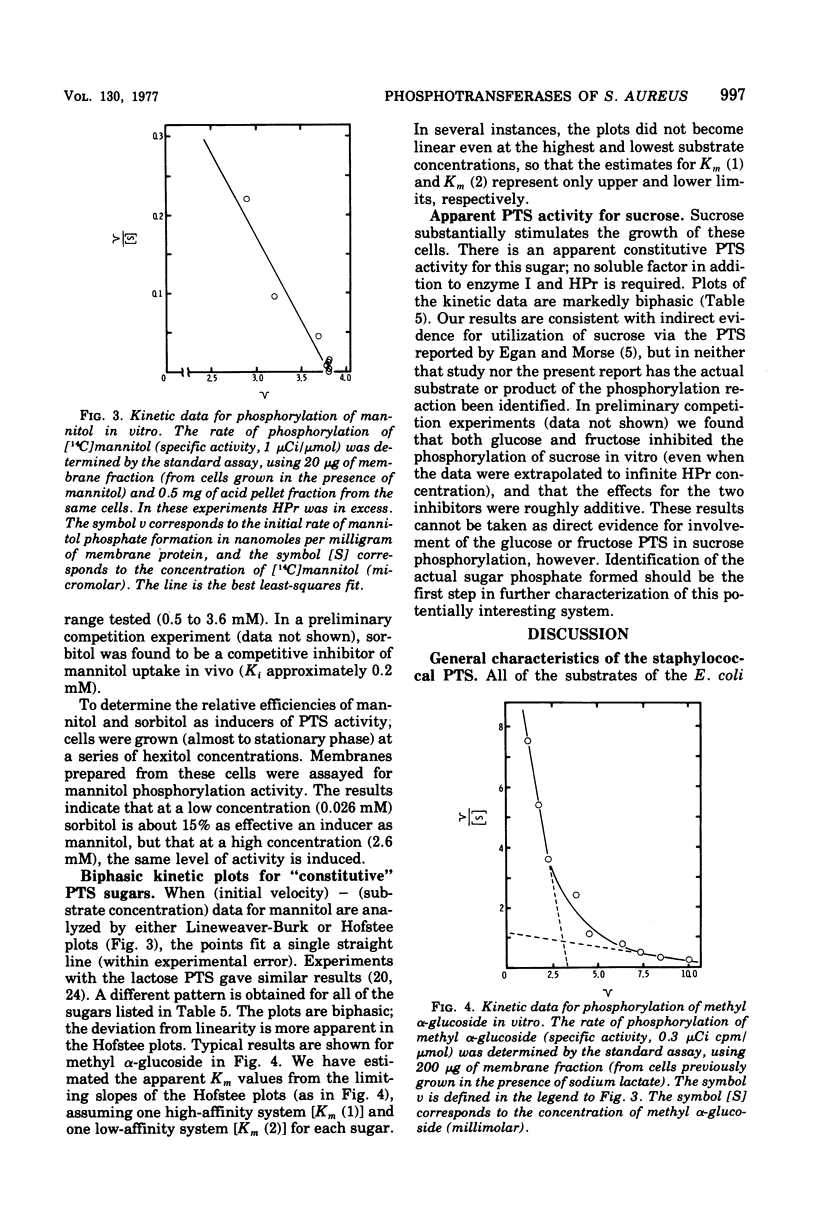
The phosphoenolpyruvate sugar phosphotransferases of Staphylococcus aureus were surveyed biochemically to determine substrate range, inducibility and constitutivity, and requirements for soluble sugar-specific proteins. The substrate range is similar to that of the phosphotransferases of enteric bacteria, but the staphylococcal mannose and sorbitol systems are very inefficient. In addition, S. qureus has phosphotransferase activities for lactose and sucrose. The systems tested fell into two broad classes. Sugars for which there was substantial constitutive activity (fructose, mannose, sucrose, and glucose and its nonmetabolized analogues) did not require sugar-specific soluble factors for phosphorylation. Only in the case of fructose did growth in the presence of these constitutive sugars induce the corresponding phosphotransferase activity to higher levels. Kinetic experiments with each of these constitutive sugars yielded biphasic Hofstee plots; i.e., the kinetics were not characteristic of single enzymes. Preliminary experiments suggest that sucrose phosphorylation may involve the glucose and/or fructose systems. Truly inducible sugar phosphotransferase systems represent a second class; those for lactose and mannitol are the only members thus far identified. These systems are absent from uninduced cells, require soluble sugar-specific factors, and exhibit linear Hofstee plots. Sorbitol is apparently transported very poorly by intact cells but is an inducer of the mannitol system; it is phosphorylated efficiently in vitro by extracts of cells grown on either hexitol, but is taken up by intact cells at 0.1% of the mannitol rate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Button D. K., Egan J. B., Hengstenberg W., Morse M. L. Carbohydrate transport in Staphylococcus aureus. IV. Maltose accumulation and metabolism. Biochem Biophys Res Commun. 1973 Jun 8;52(3):850–855. doi: 10.1016/0006-291x(73)91015-2. [DOI] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):172–183. doi: 10.1016/0926-6585(65)90101-9. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Simoni R. D., Roseman S. Sugar transport. V. A trimeric lactose-specific phosphocarrier protein of the Staphylococcus aureus phosphotransferase system. J Biol Chem. 1973 Feb 10;248(3):941–956. [PubMed] [Google Scholar]
- Hays J. B., Sussman M. L., Glass T. W. Inhibition by 6-O-tosyl galactosides of beta-galactoside phosphorylation and transport by the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1975 Nov 25;250(22):8834–8839. [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Metabolism of lactose by Staphylococcus aureus. J Bacteriol. 1968 Dec;96(6):2187–2188. doi: 10.1128/jb.96.6.2187-2188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W. Solubilization of the membrane bound lactose specific component of the staphylococcal PEP dependant phosphotransferase system. FEBS Lett. 1970 Jun 27;8(5):277–280. doi: 10.1016/0014-5793(70)80286-1. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The nature and control of carbohydrate uptake by Escherichia coli. FEBS Lett. 1976 Mar 15;63(1):3–9. doi: 10.1016/0014-5793(76)80183-4. [DOI] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Simoni R. D., Hays J. B., Roseman S. Phosphorylation of a sugar-specific protein component of the lactose transport system in Staphylococcus aureus. Biochem Biophys Res Commun. 1971 Mar 5;42(5):836–843. doi: 10.1016/0006-291x(71)90506-7. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Rose S. P., Fox C. F. The beta-glucoside system of Escherichia coli. 3. Properties of a P-HPr: beta-glucoside phosphotransferase extracted from membranes with detergent. J Supramol Struct. 1973;1(6):565–587. doi: 10.1002/jss.400010610. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Newman M. J. Direct transfer of the phosphoryl moiety of mannitol 1-phosphate to [14C]mannitol catalyzed by the enzyme II complexes of the phosphoenolpyruvate: mannitol phosphotransferase systems in Spirochaeta aurantia and Salmonella typhimurium. J Biol Chem. 1976 Jun 25;251(12):3834–3837. [PubMed] [Google Scholar]
- Simoni R. D., Hays J. B., Nakazawa T., Roseman S. Sugar transport. VI. Phosphoryl transfer in the lactose phosphotransferase system of Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):957–965. [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Simoni R. D., Smith M. F., Roseman S. Resolution of a staphylococcal phosphotransferase system into four protein components and its relation to sugar transport. Biochem Biophys Res Commun. 1968 Jun 10;31(5):804–811. doi: 10.1016/0006-291x(68)90634-7. [DOI] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Schrecker O., Lauppe H. F., Hengstenberg H. The staphylococcal PEP dependent phosphotransferase system: demonstration of a phosphorylated intermediate of the enzyme I component. FEBS Lett. 1974 May 15;42(1):98–100. doi: 10.1016/0014-5793(74)80288-7. [DOI] [PubMed] [Google Scholar]
- Wolfson E. B., Sobel M. E., Krulwich T. A. Phosphoenolpyruvate:fructose phosphotransferase activity in whole cells and membrane vesicles of Arthrobacter pyridinolis. Biochim Biophys Acta. 1973 Sep 15;321(1):181–188. doi: 10.1016/0005-2744(73)90072-7. [DOI] [PubMed] [Google Scholar]


