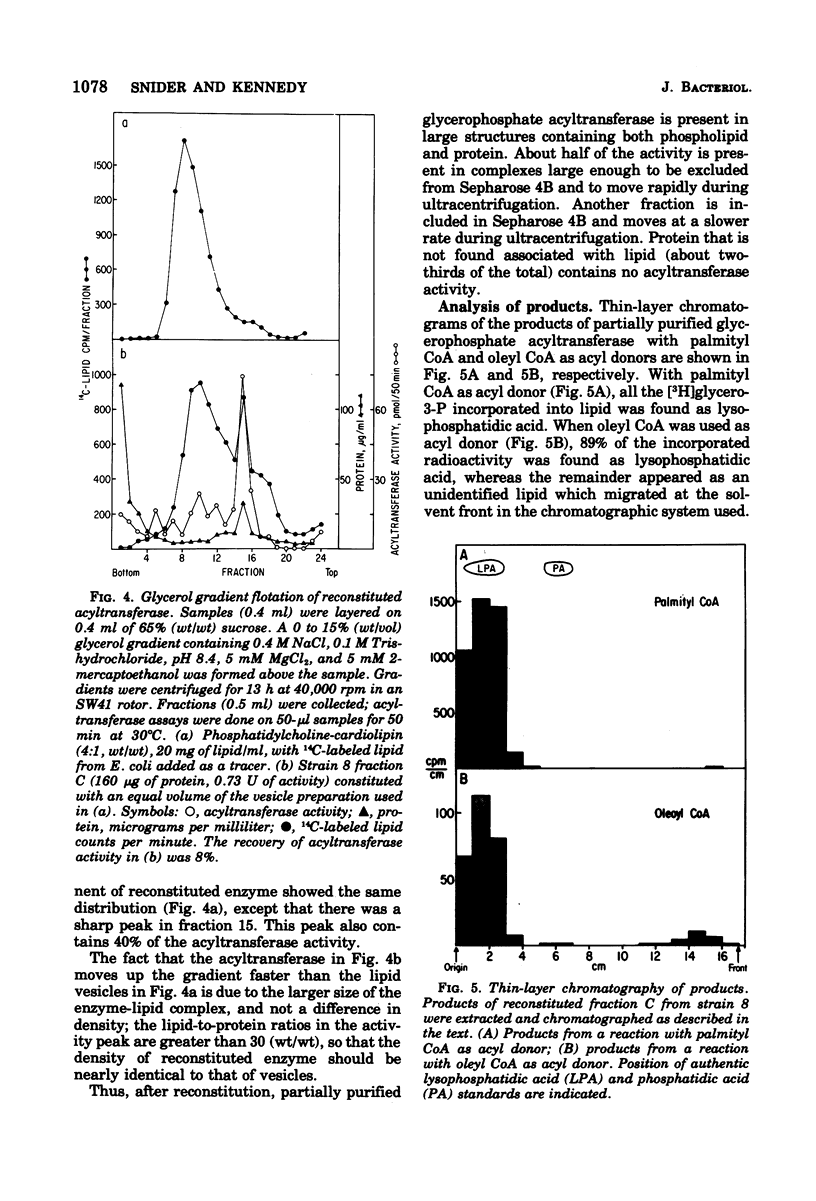
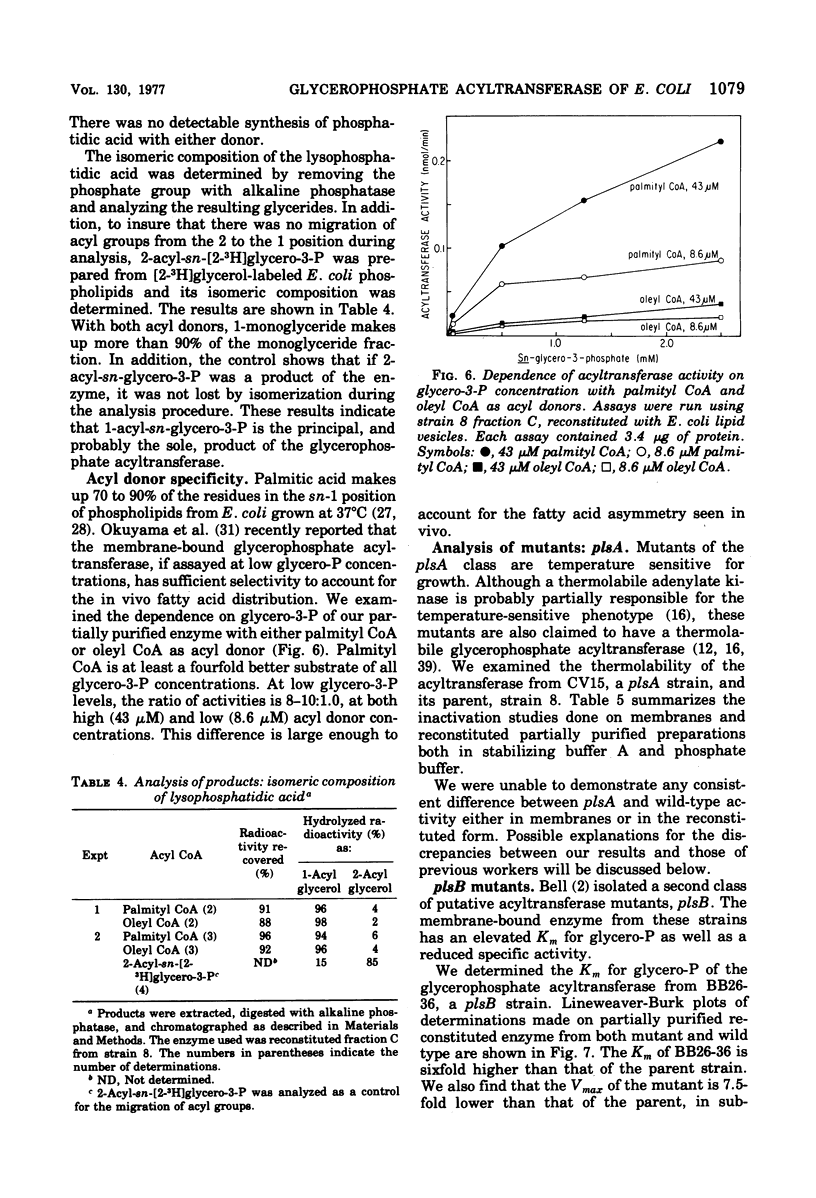
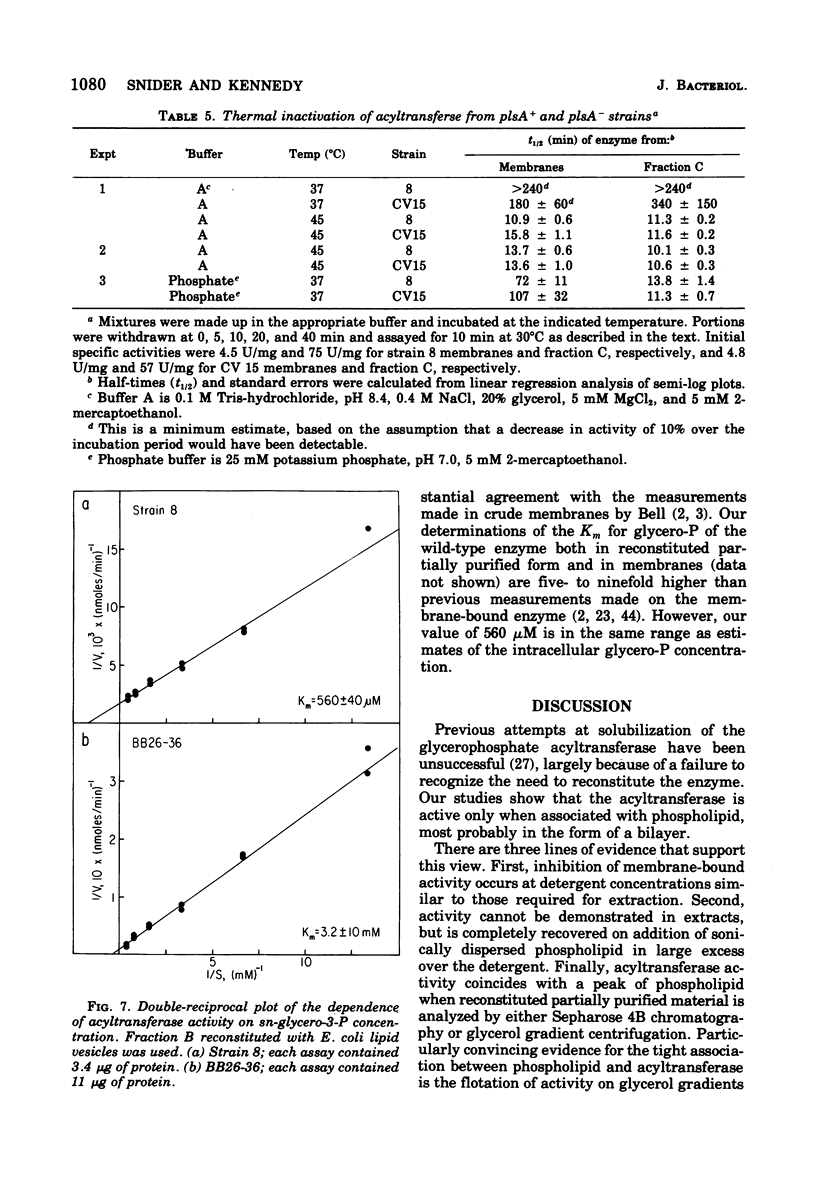
Abstract
Glycerophosphate acyltransferase, a membrane-bound enzyme catalyzing the initial step of phospholipid biosynthesis in Escherichia coli, has been extracted with Triton X-100, a nonionic detergent, and purified 20- to 40-fold. This preparation is free from lysophosphatidate acyltransferase. Glycerophosphate acyltransferase is inactive in detergent extracts, but can be reconstituted by the addition of phospholipid. Under such conditions, the enzyme is associated with phospholipid. The sole product of the reaction with acyl coenzyme A as substrate is 1-acyl-sn-glycero-3-phosphate. Furthermore, the enzyme shows a marked preference for saturated fatty acyl conenzyme A, implying that this enzyme is responsible for the predominance of saturated moieties in position 1 of E. coli phospholipids. Acyltransferase from two mutants, plsA and plsB, was partially purified and characterized. Results support the view that plsB is a structural gene for the acyltransferase, but suggest that the plsA gene product is not directly involved in phospholipid biosynthesis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aibara S., Kato M., Ishinaga M., Kito M. Changes in positional distribution of fatty acids in the phospholipids of Escherichia coli after shift-down in temperature. Biochim Biophys Acta. 1972 Jul 7;270(3):301–306. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Properties of wild type and Km defective sn-glycerol-3-phosphate acyltransferase activities. J Biol Chem. 1975 Sep 25;250(18):7147–7152. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Phosphatidyl glycerophosphate phosphatase. J Lipid Res. 1967 Sep;8(5):456–462. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974 Oct;120(1):227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Dowhan W., Wickner W. T., Kennedy E. P. Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1974 May 25;249(10):3079–3084. [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Nulty W., Vagelos P. R. Role of adenylate kinase in the regulation of macromolecular biosynthesis in a putative mutant of Escherichia coli defective in membrane phospholipid biosynthesis. J Bacteriol. 1975 Jul;123(1):128–136. doi: 10.1128/jb.123.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILDEBRAND J. G., LAW J. H. FATTY ACID DISTRIBUTION IN BACTERIAL PHOSPHOLIPIDS. THE SPECIFICITY OF THE CYCLOPROPANE SYNTHETASE REACTION. Biochemistry. 1964 Sep;3:1304–1308. doi: 10.1021/bi00897a020. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Agranoff B. W. Acyl dihydroxyacetone phosphate. Characterization of a 32P-labeled lipid from guinea pig liver mitochondria. J Biol Chem. 1968 Apr 10;243(7):1617–1622. [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Kito M., Lubin M., Pizer L. I. A mutant of Escherichia coli defective in phosphatidic acid synthesis. Biochem Biophys Res Commun. 1969 Feb 21;34(4):454–458. doi: 10.1016/0006-291x(69)90403-3. [DOI] [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Phosphatidic acid synthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1321–1327. doi: 10.1128/jb.97.3.1321-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lueking D. R., Goldfine H. The involvement of guanosine 5-diphosphate-3-diphosphate in the regulation of phospholipid biosynthesis in Escherichia coli. Lack of ppGpp inhibition of acyltransfer from acyl-ACP to sn-glycerol 3-phosphate. J Biol Chem. 1975 Jul 10;250(13):4911–4917. [PubMed] [Google Scholar]
- Luria S. E., Suit J. L., Plate C. A. Initiation of transcription is temperature-dependent in an E. coli mutant with ts adenylate kinase. Biochem Biophys Res Commun. 1975 Nov 3;67(1):353–358. doi: 10.1016/0006-291x(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Merlie J. P., Pizer L. I. Regulation of phospholipid synthesis in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1973 Oct;116(1):355–366. doi: 10.1128/jb.116.1.355-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Kelker H. C., Pullman M. E. Partial purification and properties of an acyl coenzyme A:sn-glycerol 3-phosphate acyltransferase from rat liver mitochondria. J Biol Chem. 1973 Apr 25;248(8):2845–2852. [PubMed] [Google Scholar]
- Okuyama H., Wakil S. J. Positional specificities of acyl coenzyme A: glycerophosphate and acyl coenzyme A: monoacylglycerophosphate acyltransferases in Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):5197–5205. [PubMed] [Google Scholar]
- Okuyama H., Yamada K., Ikezawa H., Wakil S. J. Factors affecting the acyl selectivities of acyltransferases in Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2487–2492. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Merlie J. P., De Leon M. P. Metabolic consequences of limited phospholipid synthesis in Escherichia coli. J Biol Chem. 1974 May 25;249(10):3212–3224. [PubMed] [Google Scholar]
- Racker E., Chien T. F., Kandrach A. A cholate-dilution procedure for the reconstitution of the Ca++ pump, 32Pi--ATP exchange, and oxidative phosphorylation. FEBS Lett. 1975 Sep 1;57(1):14–18. doi: 10.1016/0014-5793(75)80141-4. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Dowhan W., Kennedy E. P. Partial purification and characterization of cytidine 5'-diphosphate-diglyceride hydrolase from membranes of Escherichia coli. J Bacteriol. 1976 Mar;125(3):855–863. doi: 10.1128/jb.125.3.855-863.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Partial purification and properties of phosphatidylserine synthetase from Escherichia coli. J Biol Chem. 1974 Aug 25;249(16):5083–5045. [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr Acylation of sn-glycerol 3-phosphate in Escherichia coli. Study of reaction with native palmitoyl-acyl carrier protein. J Biol Chem. 1975 Nov 10;250(21):8422–8427. [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Godson G. N. Specific inhibition of phospholipid synthesis in plsA mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):136–141. doi: 10.1128/jb.125.1.136-141.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Mavis R. D., Vagelos P. R. The specific acylation of glycerol 3-phosphate to monoacylglycerol 3-phosphate in Escherichia coli. Evidence for a single enzyme conferring this specificity. J Biol Chem. 1970 Dec 10;245(23):6442–6448. [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Partial purification and properties of diglyceride kinase from Escherichia coli. Biochim Biophys Acta. 1976 Aug 23;441(2):201–212. doi: 10.1016/0005-2760(76)90163-6. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Numa S. Partial purification and properties of glycerophosphate acyltransferase from rat liver. Formation of 1-acylglycerol 3-phosphate from sn-glycerol 3-phosphate and palmityl coenzyme A. Eur J Biochem. 1972 Dec 18;31(3):565–573. doi: 10.1111/j.1432-1033.1972.tb02566.x. [DOI] [PubMed] [Google Scholar]


