Abstract
Stressed mammalian cells up-regulate heme oxygenase 1 (Hmox1; EC 1.14.99.3), which catabolizes heme to biliverdin, carbon monoxide, and free iron. To assess the potential role of Hmox1 in cellular antioxidant defense, we analyzed the responses of cells from mice lacking functional Hmox1 to oxidative challenges. Cultured Hmox1−/− embryonic fibroblasts demonstrated high oxygen free radical production when exposed to hemin, hydrogen peroxide, paraquat, or cadmium chloride, and they were hypersensitive to cytotoxicity caused by hemin and hydrogen peroxide. Furthermore, young adult Hmox1−/− mice were vulnerable to mortality and hepatic necrosis when challenged with endotoxin. Our in vitro and in vivo results provide genetic evidence that up-regulation of Hmox1 serves as an adaptive mechanism to protect cells from oxidative damage during stress.
Oxidative stress is believed to underly the etiology of numerous human conditions, including atherosclerosis, cerebral ischemia, and several neurodegenerative and neuromuscular disorders (1). Cellular antioxidants appear to be crucial for the reduction of oxidative stress and the prevention of associated pathology. Of the known enzymatic antioxidant systems, perhaps the best characterized are superoxide dismutases, catalases, and glutathione peroxidase, which directly metabolize free radical precursors (2). In addition, certain proteins of the heat shock family are strongly induced during hyperthermia and stress, and ostensibly act to maintain the structural and functional integrity of damaged proteins (3).
Mammalian heme oxygenase 1 (Hmox1), one of two isoforms of heme oxygenase (Hmox; EC 1.14.99.3) that catabolize cellular heme to biliverdin, carbon monoxide, and free iron, is also up-regulated strongly during stress, and it is considered one of the most sensitive and reliable indicators of cellular oxidative stress. Hmox1 expression is normally difficult to detect in cells other than macrophages, but it is markedly activated in virtually all cell types by initiators of stress such as hyperthermia (4), heme and metals (5, 6), oxidized lipoproteins (7), UV and visible light (8, 9), inflammatory cytokines (10, 11), and hypoxia (12), and it is found to be up-regulated in disease models such as endotoxemia and ischemia in rodents (13, 14) and in human Alzheimer disease (15). This response is explained by the multitude of stress-activated recognition sites contained within the Hmox1 promoter, including activator protein 1 sites, CCAAT/enhancer-binding proteins sites, phorbol ester response elements, heme response elements, and antioxidant response elements (16).
In analogy to heat shock regulation, several researchers have proposed that up-regulation of Hmox1 during stress is an adaptive mechanism that may protect cells from oxidative damage. Experimental evidence for this hypothesis stems from observations that cellular resistance to oxidative stress correlates positively with levels of Hmox1 expression. In fact, researchers using various in vitro or in vivo stress paradigms have found that experimental up-regulation of Hmox1 by treatment with heme or hemoglobin affords protection against subsequent oxidative challenges (17–19).
In our accompanying paper, we describe the important role of Hmox1 in adult iron homeostasis, based on the analysis of mice with targeted Hmox1 mutations (20). Here, to examine the extent to which Hmox1 participates in the stress response, we analyze the effects of in vitro oxidative challenges to cells isolated from Hmox1-deficient mice. In addition, we examine how mice lacking Hmox1 respond to oxidative stress caused by administration of endotoxin. Our results indicate that murine cells lacking Hmox1 are susceptible to the accumulation of free radicals and to oxidative injury in vitro and in vivo, thus establishing that Hmox1 is an important enzymatic antioxidant system.
MATERIALS AND METHODS
Isolation of Embryonic Fibroblasts.
Litters of Hmox1−/− and Hmox1+/− pups were obtained at embryonic day 12.5 (E12.5) after timed matings between Hmox1−/− males and Hmox1+/− females. In addition, E12.5 litters of Hmox1+/− and Hmox1+/+ pups were obtained after timed matings between Hmox1+/− males and Hmox1+/+ females. Each pup was decapitated, viscera were removed, and the remaining portion was trypsinized and plated in 2 wells of a 6-well plate. After overnight growth, the murine embryonic fibroblasts (MEFs) were expanded to 6-well plates for RNA analysis, 12-well plates for oxygen radical analysis, or 24-well plates for cytotoxicity analysis, and they were grown to confluency overnight prior to drug treatments. Concurrently, DNA was isolated from head tissue of each pup and genotyped as previously reported (20). Each experiment compared responses of one to three pups of each genotype.
Analysis of Free Radical Generation in MEFs.
Hmox1−/− and Hmox1+/− MEFs plated in 12-well plates were exposed to various oxidants for 24 hr. Cells were washed, trypsinized, and incubated with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes), which releases the fluorescent dye 2′,7′ dichlorofluorescein when oxidized, at 37°C for 20 min. Samples containing 10,000 cells were analyzed for fluorescein fluorescence by flow cytometry (Becton Dickinson). Data were collected only from live cells.
Cytotoxicity.
MEFs plated in 24-well plates were exposed to oxidants for 24 hr. For cells treated with hemin and H2O2, cells were collected and stained with trypan blue, and 250–500 cells were analyzed for viability by using a hemocytometer. For technical reasons, cytotoxicity was assessed in cells treated with CdCl2 and paraquat by measurements of lactate dehydrogenase, an indicator of lysis, in culture medium by using a kit (Boehringer Mannheim).
Lipolysaccharide (LPS) Administration.
Hmox1+/+, Hmox1+/−, and Hmox1−/− mice 6–9 weeks old were injected with LPS from Escherichia coli serotype O26:B6 (Sigma) dissolved in saline. Doses ranged from 0.5 to 25 mg/kg. Mice were monitored for signs of endotoxemia and lethality twice daily for 4 days. To examine Hmox1 mRNA induction, Hmox1+/+ mice were sacrificed 6–24 hr after injection, and peritoneal macrophages and livers were dissected for RNA isolation. For liver enzyme analyses (performed by Tufts Veterinary Diagnostic Labs), animals were bled retroorbitally 60 hr after injection and serum was isolated. When histopathology was required, three mice of each genotype representing each dose were sacrificed 60 hr after injection for organ dissection and fixation. Histology was performed as described (20).
Nitrite generation was analyzed from in vitro-stimulated macrophages as described (21). Free radical generation was measured by incubating untreated or phorbol 12-myristate 13-acetate-treated resident or in vivo-stimulated peritoneal macrophages with 10 μM of the free radical-activated dye 2′,7′-dichlorodihydrofluorescein diacetate for 20 min, followed by analysis of mean cellular fluorescein fluorescence by flow cytometry (Becton Dickinson). Gating was adjusted so that 10,000 live macrophages per sample were assayed.
RESULTS
Decreased Stress Defense in Hmox1−/− Embryonic Fibroblasts.
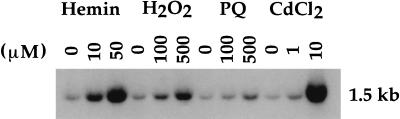
MEFs were utilized as an in vitro system for testing the requirement of Hmox1 in stress defense. We first examined whether MEFs isolated from wild-type E12.5 embryos demonstrate induction of Hmox1 mRNA. When exposed for 6 hr to the oxidants hemin (a substrate of Hmox), hydrogen peroxide (H2O2), the superoxide generator paraquat, or cadmium chloride (CdCl2), a dose-dependent up-regulation of Hmox1 mRNA transcription was observed (Fig. 1). The strongest induction was observed in cells exposed to 50 μM hemin or 10 μM CdCl2 (Fig. 1, lanes 3 and 12).
Figure 1.
Induction of Hmox1 mRNA in oxidant-exposed embryonic fibroblasts. Fibroblasts isolated from E12.5 Hmox1+/+ embryos and plated in 6-well plates were untreated or exposed to the indicated concentrations of hemin, H2O2, paraquat (PQ), or CdCl2. After 6 hr, total RNA was extracted, Northern-blotted, and hybridized with a rat Hmox1 cDNA probe, which recognizes a major mRNA band of approximately 1.5 kb (22).
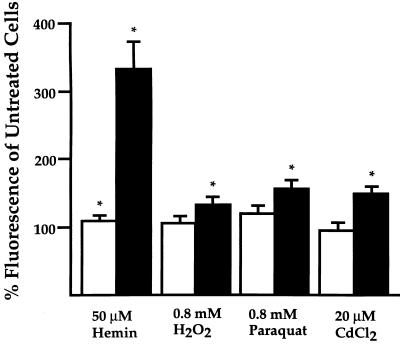
To determine whether the presence or absence of this particular stress response affects the magnitude of oxidative stress, we measured free radical generation in MEFs that had been isolated from either heterozygous (Hmox1+/−) or homozygous (Hmox1−/−) mutant E12.5 mice and exposed for 24 hr to the oxidants mentioned above. Hemin applied to Hmox1+/− cells at 50 μM caused a significant but minor 11% increase in free radical generation over untreated cells, based on quantification of a oxidation-activated fluorescent dye. In contrast, hemin applied at 50 μM to Hmox1−/− MEFs resulted in an increase in free radical generation of 232% over untreated cells. Similar exposure of Hmox1+/− cells to 0.8 mM H2O2, 0.8 mM paraquat, or 20 μM CdCl2 revealed insignificant changes in free radical production of +5%, +20%, and −5%, respectively, while Hmox1−/− MEFs treated with these oxidants incurred significant increases of 31%, 53%, and 48% over untreated cells (Fig. 2).
Figure 2.
Enhanced free radical production in Hmox1−/− embryonic fibroblasts. Percentage mean cellular fluorescence of oxidant-treated cells compared with that of untreated cells, as detected by flow cytometry. Fibroblasts plated in 12-well plates were untreated or exposed to the indicated oxidants for 24 hr and incubated for 20 min with an oxidation-activated dye. Open bars represent Hmox1+/− percentages, while closed bars represent Hmox1−/− percentages. Data are shown mean ± SEM. Each bar represents combined data from 4–5 experiments sampled in duplicate. ∗, Significant differences were observed between treated and untreated fibroblasts of that genotype (P < 0.05).
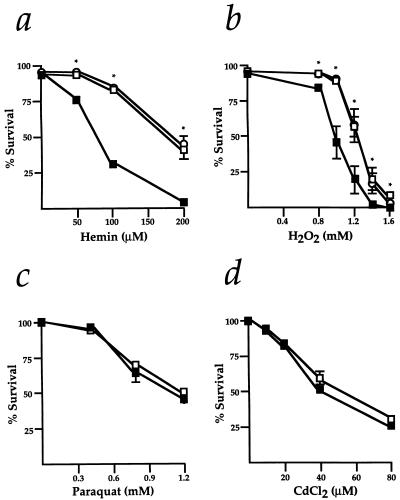
We next studied the survival of Hmox1+/− and Hmox1−/− MEFs exposed to several doses of each oxidant. Both hemin and H2O2 had significantly more severe effects on the viability of Hmox1−/− MEFs. For instance, hemin concentrations of 100 μM and 200 μM resulted in 84% and 38% survival of Hmox1+/− cells, but only 32% and 4% survival of Hmox1−/− cells, respectively (Fig. 3a). Similarly, H2O2 concentrations of 1 mM and 1.2 mM resulted in 88% and 56% survival of Hmox1+/− cells, compared with only 46% and 19% survival of Hmox1−/− cells (Fig. 3b). Hmox1+/− cell viability was comparable to Hmox1+/+ cell viability when the cells were exposed to hemin or H2O2 (Fig. 3 a and b). When treated with different concentrations of paraquat or CdCl2, Hmox1−/− cells showed vulnerability similar to that of Hmox1+/− cells (Fig. 3 c and d). From our in vitro experiments, we deduced that optimal maintenance of free radical formation and cell viability during direct exposure of MEFs to certain oxidants requires the expression of functional Hmox1.
Figure 3.
Survival of Hmox1−/− embryonic fibroblasts exposed to oxidants. (a–d) Fibroblasts plated in 24-well plates were exposed for 24 hr to several doses each of hemin (a), H2O2 (b), paraquat (c), or CdCl2 (d). Cell survival was assayed by trypan blue exclusion or lactate dehydrogenase release. ○, Hmox1+/+ values; □, Hmox1+/− values; and ▪, Hmox1−/− values. Data are shown mean ± SEM. For each genotype and each drug tested, survival data are from 3–6 experiments sampled in duplicate or triplicate for each drug concentration. ∗, Significant differences were observed between Hmox1+/− and Hmox1−/− cell survival (P < 0.05).
Abnormal Responses of Hmox1−/− Mice to Endotoxin.
To address whether Hmox1−/− cells also show hypersensitivity to in vivo oxidative challenges, we chose endotoxemia, a mouse model of sepsis in humans. Previous studies have shown that pathology resulting from high doses of endotoxin is attributable to oxidative mechanisms, such as phagocytic cell oxidative respiratory bursts that may damage vascular endothelial cells (23, 24), major organ hypoxia due to vasorelaxation (25), and the direct oxidative effects of endotoxin on hepatocytes (26, 27).
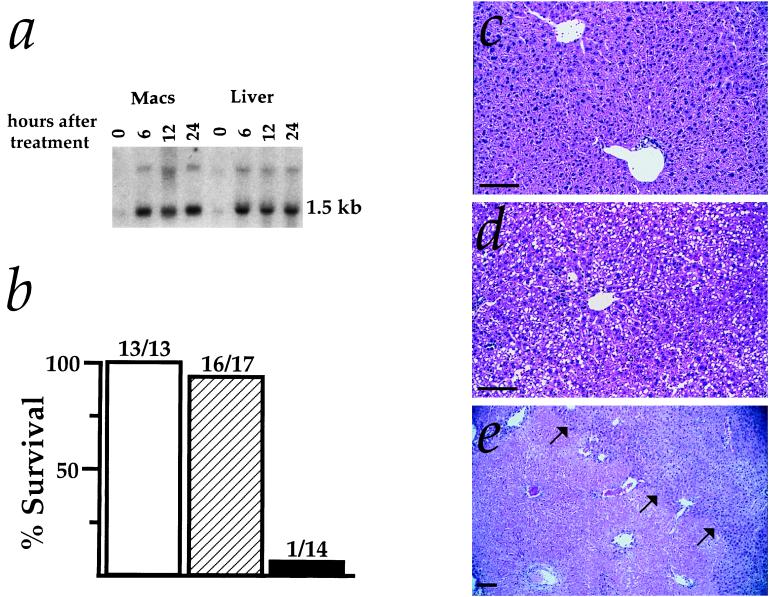
Adult Hmox1−/− mice show a variety of disease symptoms, including anemia, iron-loading, and chronic inflammation, that are not yet detectable at 6–9 weeks of age but are first evident by around 20 weeks of age (20). Therefore, we examined the responses of 6- to 9-week-old Hmox1+/+, Hmox1+/−, and Hmox1−/− mice to inflammatory stress caused by endotoxin administration, which induced Hmox1 mRNA in both peritoneal macrophages and livers of Hmox1+/+ mice (Fig. 4a). In tests of survival, Hmox1−/− mice were clearly more vulnerable than Hmox1+/− and Hmox1+/+ mice to a high dose (25 mg/kg) of LPS administered intraperitoneally (Fig. 4b).
Figure 4.
Responses of Hmox1−/− mice to endotoxemia. (a) Induction of Hmox1 mRNA in Hmox1+/+ mice injected with 2.5 mg/kg LPS. At 0, 6, 12, and 24 hr, total RNA was extracted from isolated peritoneal macrophages (Macs) and liver, Northern-blotted, and hybridized with a rat Hmox1 cDNA probe. (b) Survival of 6- to 9-week-old Hmox1+/− (hatched bar) and Hmox1−/− (closed bar) littermates as well as similarly aged Hmox1+/+ mice (open bar) after intraperitoneal injection with 25 mg/kg LPS. The data are combined from five independent experiments using 44 female mice. Deaths occurred 2–3 days after injection in all cases. (c) High-magnification view of hematoxylin and eosin-stained liver section from 6- to 9-week-old Hmox1−/− mice 60 hr after being given 1 mg/kg LPS. These histological results were indistinguishable from those of Hmox1+/− mice given 1, 5, or 25 mg/kg LPS, with no signs of toxicity. (d) High-magnification view of liver pathology 60 hr after administration of 5 mg/kg LPS to 6- to 9-week-old Hmox1−/− mice. Note hepatocellular fatty vacuoles indicative of toxicity. (e) Low-magnification view of liver pathology 60 hr after administration of 25 mg/kg LPS to 6- 9-week-old Hmox1−/− mice. These mice were moribund and near death when tissues were harvested. Note the large, anuclear necrotic infarct in the lower left portion of the photo (arrows point from necrotic tissue toward region of viable tissue). (All magnification bars = 100 μm.)
To determine if any organ(s) was especially susceptible to LPS treatment in Hmox1−/− animals, we examined the pathological effects of this high dose and of two lower doses of LPS. Hmox1+/− mice given 1, 5, or 25 mg/kg LPS had no significant histopathological abnormalities (Fig. 4c). However, LPS had conspicuous effects on Hmox1−/− hepatic tissue. A low dose of 1 mg/kg did not result in histopathological anomalies (Fig. 4c), but it caused increases in serum liver enzyme levels significantly greater than in Hmox1+/− animals (data not shown). Furthermore, livers from Hmox1−/− animals given an intermediate dose of 5 mg/kg LPS showed hepatocellular vacuoles indicative of toxicity (Fig. 4d). Livers harvested from moribund Hmox1−/− mice that had received the high dose of 25 mg/kg LPS had massive necrotic infarcts, evident in the lower left portion of Fig. 4e by a large faint-staining region.
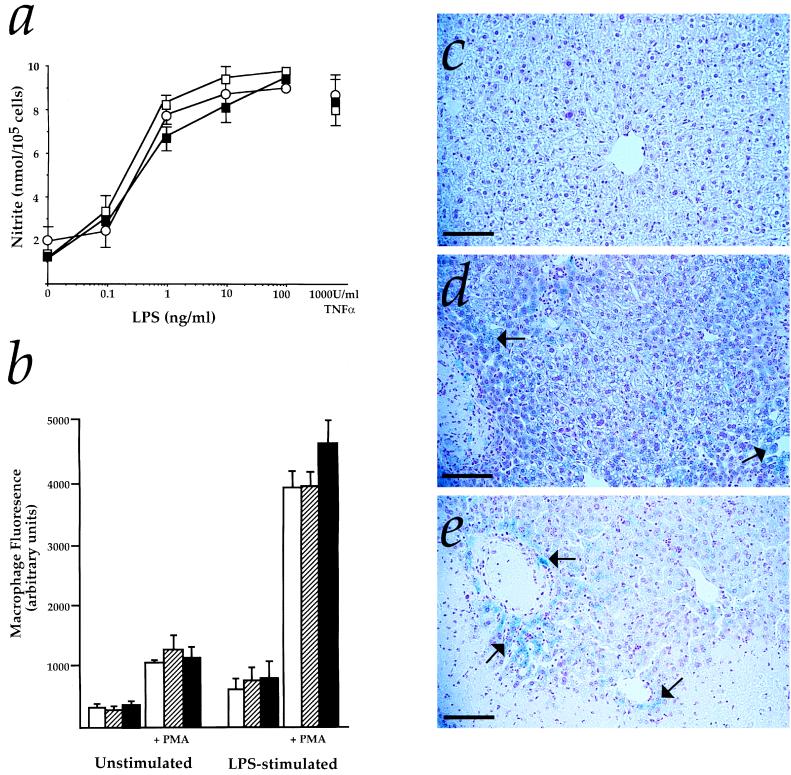
Consistent with the idea that this pathology was due to hepatic rather than macrophage defects, both nitric oxide and total free radical production in LPS-stimulated Hmox1−/− macrophages were normal (Fig. 5 a and b). Also, there appeared to be specific defects of hepatic iron metabolism in endotoxemic Hmox1−/− mice. No evidence of iron-loading was observed in livers isolated from Hmox1+/− mice 3 days after administration of 1, 5, or 25 mg/kg LPS, nor in Hmox1−/− animals given an LPS dose of 1 mg/kg (Fig. 5c). However, Hmox1−/− mice that were given 5 mg/kg LPS displayed diffuse but widespread iron-loading in hepatocytes (Fig. 5d). Moreover, Hmox1−/− mice administered 25 mg/kg LPS developed what appeared to be even more severe iron-loading, with hepatocellular necrotic foci in close proximity to areas of heavy iron deposition (Fig. 5e). Thus, LPS had dose-dependent effects on both hepatic iron-loading and injury, exclusively in Hmox1−/− mice. Therefore, we suspect that iron deposition contributed to hepatic injury in LPS-treated Hmox1−/− animals.
Figure 5.
Normal macrophage reponses but atypical hepatic iron-loading in Hmox1−/− mice. (a) Nitrite measured in peritoneal macrophages cultured for 48 hr with 50 units/ml interferon γ and the indicated concentrations of LPS or tumor necrosis factor α (TNFα). ○, Hmox1+/+values; □, Hmox1+/− values; and ▪, Hmox1−/− values. Data are shown mean ± SEM from three experiments with each drug concentration sampled in triplicate. (b) Fluorescein fluoresence in macrophages isolated from untreated mice or mice treated with 5 μg LPS and 1,000 units of interferon γ, incubated with or without 100 ng/ml phorbol 12-myristate 13-acetate (PMA) and with a free radical-activated dye for 20 min, and analyzed by flow cytometry. Open bars represent Hmox1+/+values, hatched bars represent Hmox1+/− values, and closed bars represent Hmox1−/− values. Data are shown mean ± SEM from three experiments with each treatment sampled in duplicate. (c) Representative liver section from Hmox1−/− mice given 1 mg/kg LPS stained with Prussian blue to detect iron. These histological results were indistinguishable from those of Hmox1+/− mice treated with 1, 5, or 25 mg/kg LPS, in that no significant iron staining was observed. (d) Liver section of Hmox1−/− mouse given 5 mg/kg LPS, stained with Prussian blue. Note the diffuse blue iron staining in many of the hepatocytes (arrows). (e) Liver section of Hmox1−/− mouse given 25 mg/kg LPS, stained with Prussian blue. Blue iron staining (arrows) is visible in viable hepatocytes adjacent to a necrotic focus (along the bottom edge of the photo). (All magnification bars = 100 μm.)
As an important supplement to our in vitro data, these results demonstrate that Hmox1−/− tissues are exquisitely sensitive to endotoxemic stress. We conclude from our findings that expression of functional Hmox1 is essential for resistance to oxidative damage.
DISCUSSION
In the accompanying paper, we show that oxidation of macromolecules and tissue injury arise spontaneously in Hmox1−/− mice, substantiating the idea that Hmox-1 may normally perform an antioxidant role (20). Here, in further addressing this idea, we examined the responses of Hmox1−/− cells to in vitro and in vivo oxidative challenges. First, we demonstrated increased free radical production and reduced survival in cultured Hmox1−/− MEFs exposed to several oxidants. Subsequently, we showed that Hmox1−/− mice are markedly sensitive to hepatic injury and mortality caused by oxidative challenges with endotoxin.
Hmox1 Is an Antioxidant Defense Enzyme.
A wide variety of stress inducers cause robust up-regulation of Hmox1 activity in mammalian cells. Many researchers have suggested that this response may afford protection from oxidative damage and have proposed several mechanisms whereby Hmox1 activity could provide this protection, including production of the antioxidants biliverdin and bilirubin (28), depletion of the oxidant heme (29), elevation of intracellular free iron levels to facilitate ferritin up-regulation (30, 31), and regulation of vascular tension through carbon monoxide generation (32, 33). On the other hand, other groups have suggested that Hmox1 up-regulation is purely correlative without functions related to modulation of oxidative damage (34). Still others have postulated that positive effects of Hmox1 activity on intracellular free iron levels may even enhance the consequences of oxidative stress (35).
By revealing the increased vulnerability of Hmox1-deficient cells to oxidative stress, our results confirm the hypothesis that Hmox1 activity supplies protective effects. Since both free radical generation and cytotoxicity are markedly enhanced in Hmox1−/− MEFs after incubation with the Hmox substrate and oxidant hemin, we infer that Hmox1 performs an important in vivo antioxidant function purely by depletion of heme. This may be most pertinent in vascular cells, or in hemorrhaged tissues, where deleterious heme is abundant. Notably, we also observed an increased sensitivity of Hmox1−/− cells to H2O2, paraquat, and CdCl2. We do not know the mechanism(s) by which Hmox1 normally protects against these oxidative insults. However, from our data, it is apparent that MEFs have adequate antioxidant systems to compensate and protect viability when exposed to paraquat and CdCl2 in the absence of functional Hmox1, even though abnormally high free radical generation occurs.
We found it especially interesting that endotoxin administration resulted in a rapid hepatic iron-loading in Hmox1−/− mice, the extent of which correlated with the severity of hepatic injury and incidence of mortality. To our knowledge, hepatic iron-loading after LPS administration has not been previously reported in animal models of endotoxemia. It might be relevant that other researchers have found reductions in hepatocellular heme levels concurrent with increases in ferritin iron levels immediately after induction of systemic inflammation in rats (36). Therefore, up-regulation of Hmox1 during inflammatory stress might be a homeostatic device that redirects heme iron to the extracellular space and thereby attenuates the accumulation of intracellular iron. In agreement with this idea, data in our accompanying paper indicate that heme catabolism by Hmox1 is important for reducing intracellular storage iron levels and maintaining blood iron levels (20). In any case, our results illustrate that proper regulation of iron levels is important during inflammatory stress.
Functions of Hmox1 During Disease.
Previously, it was shown that the pretreatment of rats with hemoglobin both up-regulated Hmox1 mRNA and protected from inflammatory injury during endotoxemia (18). Also, in a rodent model of renal failure after experimental rhabdomyolysis, similar up-regulation of Hmox1 before experimental insults reduced subsequent injury (19). Our results confirm these findings and suggest that Hmox1 may in fact be the sole mediator of this protection. Therefore, induction of Hmox1 expression might constitute an effective clinical defense against trauma associated with sepsis and renal failure. Hmox1-deficient mice should also be useful in testing the participation of Hmox1 in stress defense for other models of human conditions.
Numerous Roles of Hmox.
In summary, we have demonstrated by analysis of Hmox1-deficient mice in this and the accompanying paper (20) that Hmox1 is important for mammalian iron homeostasis and for rapid protection of cells from potential oxidative damage during stress. Furthermore, recent studies utilizing pharmacological Hmox inhibitors and analyses of mice lacking the other Hmox isoform, Hmox2, have provided evidence suggesting that the carbon monoxide product of the Hmox reaction participates in a variety of second messenger signaling systems (refs. 32, 37, and 38; R. Zakary, K.D.P., S. R. Jaffrey, C. D. Ferris, S.T., and S. H. Snyder, unpublished work). That Hmox isoforms have roles in these anatomically and functionally diverse processes illustrates the physiological versatility of this single enzymatic reaction.
Acknowledgments
We thank W. Haas and T. McHugh for critique of the manuscript. S.T. was supported by a grant from the National Institutes of Health (RO1-NS32925-03) and a gift from the Shionogi Institute for Medical Science.
ABBREVIATIONS
- Hmox
heme oxygenase
- E12.5
embryonic day 12.5
- MEFs
murine embryonic fibroblasts
- LPS
lipopolysaccharide
References
- 1.Halliwell B, Gutteridge J M C. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 2.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 3.Parsell D A, Lindquist S. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 4.Ewing J F, Maines M D. Proc Natl Acad Sci USA. 1991;88:5364–5368. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibahara S, Yoshida T, Kikuchi G. Arch Biochem Biophys. 1978;188:243–250. doi: 10.1016/s0003-9861(78)80006-x. [DOI] [PubMed] [Google Scholar]
- 6.Taketani S, Kohno H, Yoshinaga T, Tokunaga R. Biochem Int. 1988;17:665–672. [PubMed] [Google Scholar]
- 7.Siow R C, Ishii T, Taketani S, Leske D S, Sweiry J H, Pearson J D, Bannai S, Mann G E. FEBS Lett. 1995;368:239–242. doi: 10.1016/0014-5793(95)00650-x. [DOI] [PubMed] [Google Scholar]
- 8.Keyse S M, Tyrrell R M. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutty R K, Kutty G, Wiggert B, Chader G J, Darrow R M, Organisciak D T. Proc Natl Acad Sci USA. 1995;92:1177–1181. doi: 10.1073/pnas.92.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzardini M, Terao M, Falciani F, Cantoni L. Biochem J. 1993;290:343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutty R K, Naginemi G N, Kutty G, Hooks J J, Chader G J, Wiggert B. J Cell Physiol. 1994;159:371–378. doi: 10.1002/jcp.1041590221. [DOI] [PubMed] [Google Scholar]
- 12.Murphy B J, Laderoute K R, Short S M, Sutherland R M. Br J Cancer. 1991;64:69–73. doi: 10.1038/bjc.1991.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzardini M, Carelli M, Cabello Porras M R, Cantoni L. Biochem J. 1994;304:477–483. doi: 10.1042/bj3040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda A, Onododera H, Sugimoto A, Itoyama Y, Kogure K, Shibahara S. Brain Res. 1994;666:120–124. doi: 10.1016/0006-8993(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 15.Yan S D, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt A M. Nature (London) 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 16.Inamdar N M, Ahn Y I, Alam J. Biochem Biophys Res Commun. 1996;221:570–576. doi: 10.1006/bbrc.1996.0637. [DOI] [PubMed] [Google Scholar]
- 17.Balla J, Jacob H S, Balla G, Nath K, Eaton J W, Vercellotti G M. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otterbein L, Sylvester S L, Choi A M. Am J Respir Cell Mol Biol. 1995;13:595–601. doi: 10.1165/ajrcmb.13.5.7576696. [DOI] [PubMed] [Google Scholar]
- 19.Nath K A, Balla G, Vercellotti G M, Balla J, Jacob H S, Levitt M D, Rosenberg M E. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poss K D, Tonegawa S. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green S J, Aniagolu J, Raney J J. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1995. pp. 14.5.1–14.5.11. [Google Scholar]
- 22.Shibahara S, Muller R, Taguchi H, Yoshida T. Proc Natl Acad Sci USA. 1985;82:7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner S J C, Libby P. J Immunol. 1989;142:100–109. [PubMed] [Google Scholar]
- 24.Schletter J, Heine H, Ulmer A J, Rietschel E T. Arch Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 25.Cain S M. Adv Exp Med Biol. 1992;317:35–45. doi: 10.1007/978-1-4615-3428-0_4. [DOI] [PubMed] [Google Scholar]
- 26.Nolan J P. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- 27.Bautista A P, Meszaros K, Bojta J, Spitzer J J. J Leukocyte Biol. 1990;48:123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- 28.Stocker R, Yamamoto Y, McDonagh A F, Glazer A N, Ames B N. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 29.Hunt R C, Handy I, Smith A. J Cell Physiol. 1996;168:81–86. doi: 10.1002/(SICI)1097-4652(199607)168:1<81::AID-JCP10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Eisenstein R S, Garcia-Mayol D, Pettingell W, Munro H N. Proc Natl Acad Sci USA. 1991;88:688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vile G F, Basu-Modak S, Waltner C, Tyrrell R M. Proc Natl Acad Sci USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zakhary R, Gaine S P, Dinerman J L, Ruat M, Flavahan N A, Snyder S H. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A, Kim Y, Matas A, Alam J, Nath K A. Transplantation. 1996;61:93–98. doi: 10.1097/00007890-199601150-00019. [DOI] [PubMed] [Google Scholar]
- 34.Nutter L M, Sierra E E, Ngo E O. J Lab Clin Med. 1994;123:506–514. [PubMed] [Google Scholar]
- 35.Van Lenten B J, Prieve J, Navab M, Hama S, Lusis A J, Fogelman A M. J Clin Invest. 1995;95:2104–2110. doi: 10.1172/JCI117898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hershko C, Cook J D, Finch C A. Br J Haematol. 1974;28:67–75. doi: 10.1111/j.1365-2141.1974.tb06640.x. [DOI] [PubMed] [Google Scholar]
- 37.Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 38.Nathanson J A, Scavone C, Scanlon C, McKee M. Neuron. 1995;14:781–794. doi: 10.1016/0896-6273(95)90222-8. [DOI] [PubMed] [Google Scholar]