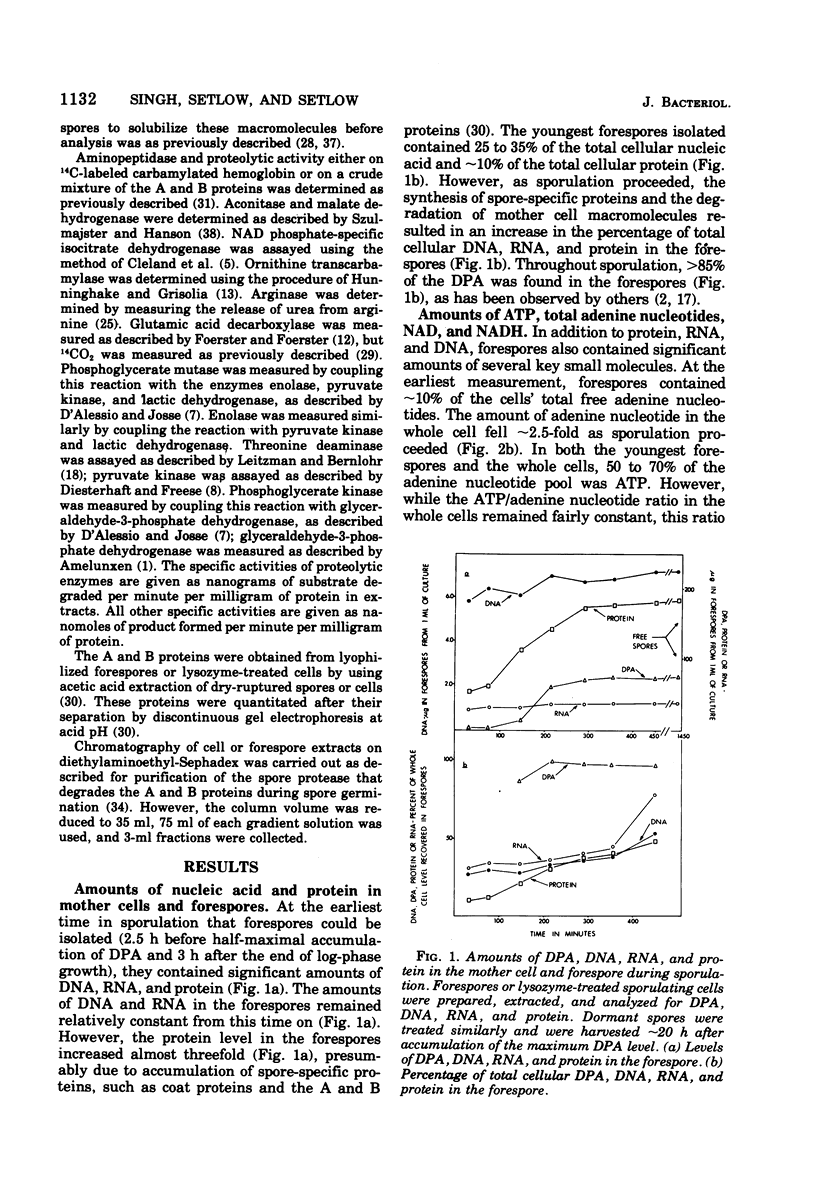
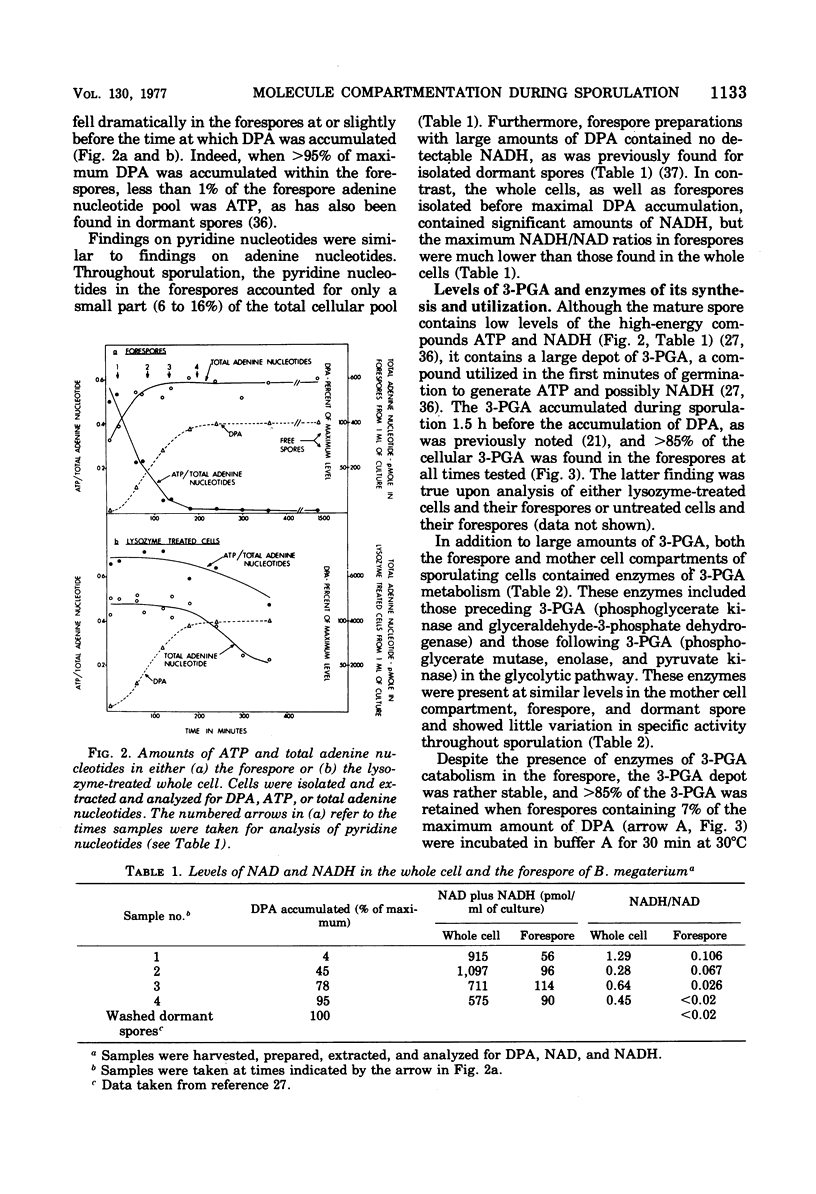
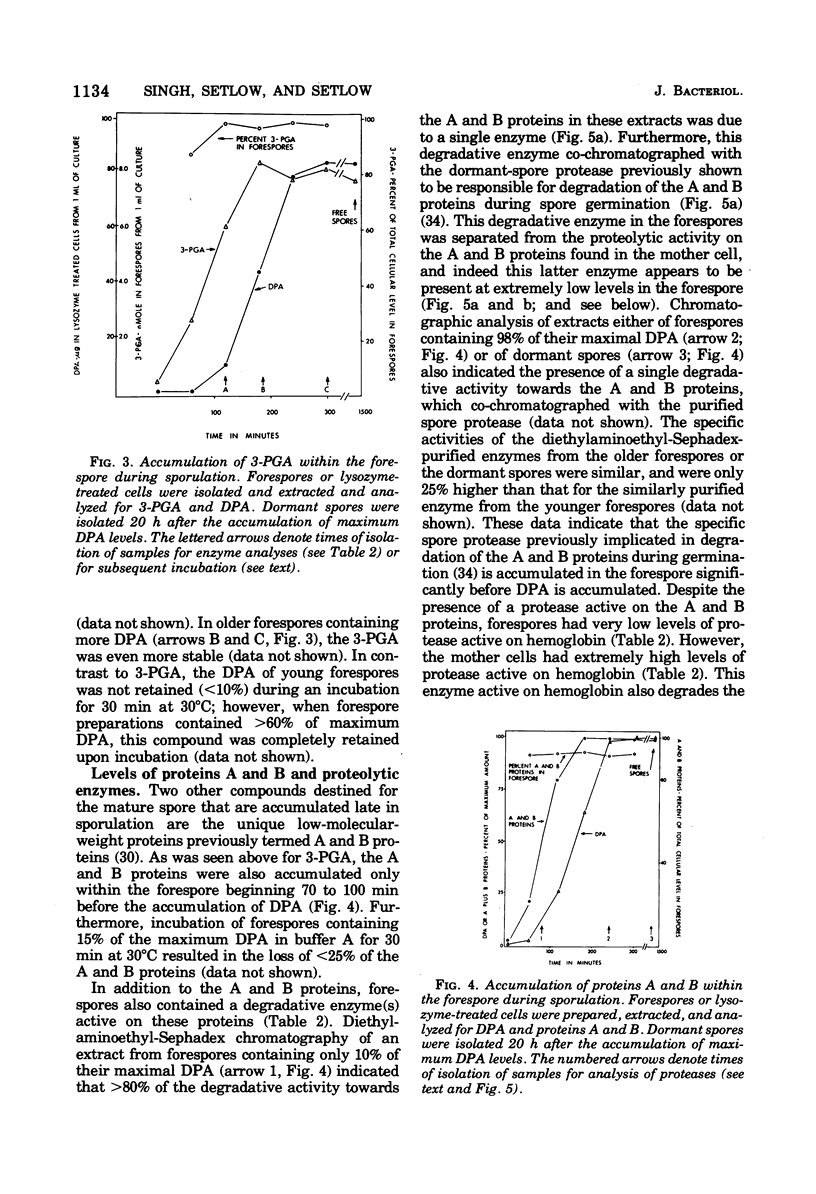
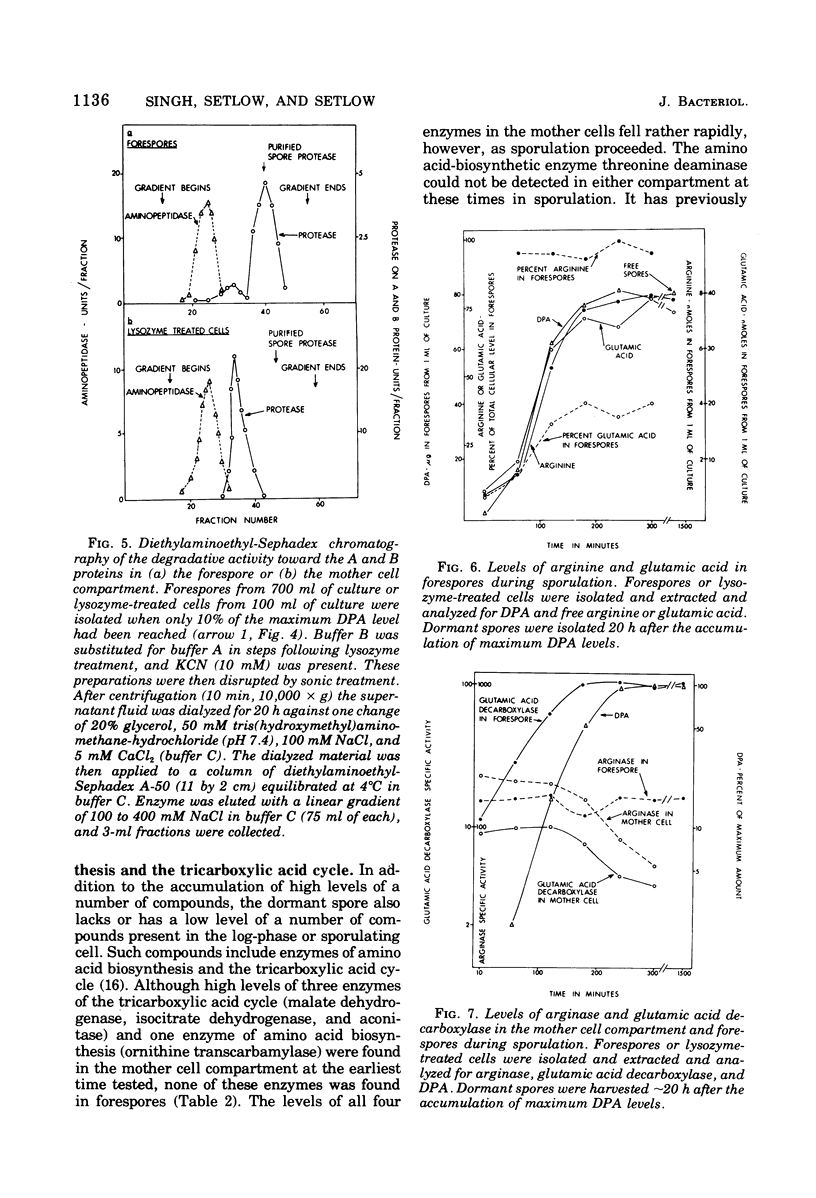
Abstract
We have determined the amounts of a number of small molecules and enzymes in the mother cell compartment and the developing forespore during sporulation of Bacillus megaterium. Significant amounts of adenosine 5′-triphosphate and reduced nicotinamide adenine dinucleotide were present in the forespore compartment before accumulation of dipicolinic acid (DPA), but these compounds disappeared as DPA was accumulated. 3-Phosphoglyceric acid (3-PGA) accumulated only within the developing forespore, beginning 1 to 2 h before DPA accumulation. Throughout its development the forespore contained constant levels of enzymes of both 3-PGA synthesis (phosphoglycerate kinase and glyceraldehyde-3-phosphate dehydrogenase) and 3-PGA utilization (phosphoglycerate mutase, enolase, and pyruvate kinase) at levels similar to those in the mother cell and the dormant spore. Despite the presence of enzymes for 3-PGA utilization, this compound was stable within isolated forespores. Two acid-soluble proteins (A and B proteins) also accumulated only in the forespore, beginning 1 to 2 h before DPA accumulation. At this time the specific protease involved in degradation of the A and B proteins during germination also appeared, but only in the forespore compartment. Nevertheless, the A and B proteins were stable within isolated forespores. Arginine and glutamic acid accumulated within the forespore in parallel with DPA accumulation. The forespore also contained the enzyme arginase at a level similar to that in the mother cell and a level of glutamic acid decarboxylase 2- to 25-fold higher than that in the mother cell, depending on when in sporulation the forespores were isolated. The specific activities of several other enzymes (protease active on hemoglobin, ornithine transcarbamylase, malate dehydrogenase, aconitase, and isocitrate dehydrogenase) in forespores were about 10% or less of the values in the mother cell. Aminopeptidase was present at similar levels in both compartments; threonine deaminase was not found in either compartment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R. E. Glyceraldehyde-3-phosphate dehydrogenase from Bacillus stearothermophilus. Methods Enzymol. 1975;41:268–273. doi: 10.1016/s0076-6879(75)41061-8. [DOI] [PubMed] [Google Scholar]
- Andreoli A. J., Suehiro S., Sakiyama D., Takemoto J., Vivanco E., Lara J. C., Klute M. C. Release and recovery of forespores from Bacillus cereus. J Bacteriol. 1973 Sep;115(3):1159–1166. doi: 10.1128/jb.115.3.1159-1166.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alession G., Josse J. Phosphoglycerate kinase and phosphoglyceromutase from Escherichia coli. Methods Enzymol. 1975;42:139–144. doi: 10.1016/0076-6879(75)42107-3. [DOI] [PubMed] [Google Scholar]
- Diesterhaft M., Freese E. Pyruvate kinase of bacillus subtilis. Biochim Biophys Acta. 1972 May 12;268(2):373–380. doi: 10.1016/0005-2744(72)90332-4. [DOI] [PubMed] [Google Scholar]
- Foerster C. W., Foerster H. F. Glutamic acid decarboxylase in spores of Bacillus megaterium and its possible involvement in spore germination. J Bacteriol. 1973 Jun;114(3):1090–1098. doi: 10.1128/jb.114.3.1090-1098.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake D., Grisolia S. A sensitive and convenient micromethod for estimation of urea, citrulline, and carbamyl derivatives. Anal Biochem. 1966 Aug;16(2):200–205. doi: 10.1016/0003-2697(66)90147-3. [DOI] [PubMed] [Google Scholar]
- Kato T., Berger S. J., Carter J. A., Lowry O. H. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973 May;53(1):86–97. doi: 10.1016/0003-2697(73)90409-0. [DOI] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leitzmann C., Bernlohr R. W. Threonine dehydratase of Bacillus licheniformis. I. Purification and properties. Biochim Biophys Acta. 1968 Feb 5;151(2):449–460. doi: 10.1016/0005-2744(68)90113-7. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem. 1970 Mar 10;245(5):1137–1145. [PubMed] [Google Scholar]
- Oh Y. K., Freese E. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):739–746. doi: 10.1128/jb.127.2.739-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Setlow P. Polyamine levels during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1974 Mar;117(3):1171–1177. doi: 10.1128/jb.117.3.1171-1177.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Setlow P. Protease and peptidase activities in growing and sporulating cells and dormant spores of Bacillus megaterium. J Bacteriol. 1975 May;122(2):642–649. doi: 10.1128/jb.122.2.642-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J Biol Chem. 1975 Jan 25;250(2):631–637. [PubMed] [Google Scholar]
- Setlow P. Purification and properties of a specific proteolytic enzyme present in spores of Bacillus magaterium. J Biol Chem. 1976 Dec 25;251(24):7853–7862. [PubMed] [Google Scholar]
- Setlow P. Purification and properties of some unique low molecular weight basic proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8168–8173. [PubMed] [Google Scholar]
- Setlow P. Spermidine biosynthesis during germination and subsequent vegetative growth of Bacillus megaterium spores. J Bacteriol. 1974 Oct;120(1):311–315. doi: 10.1128/jb.120.1.311-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


