Abstract
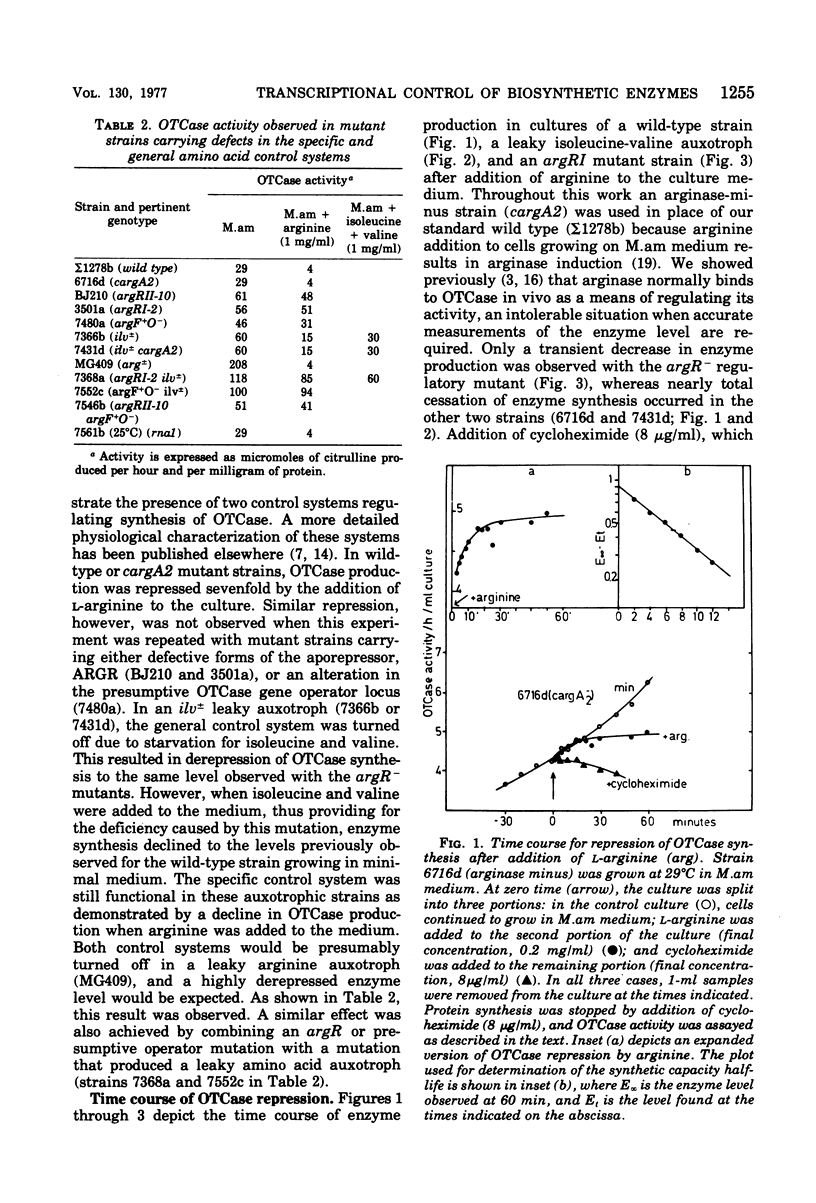
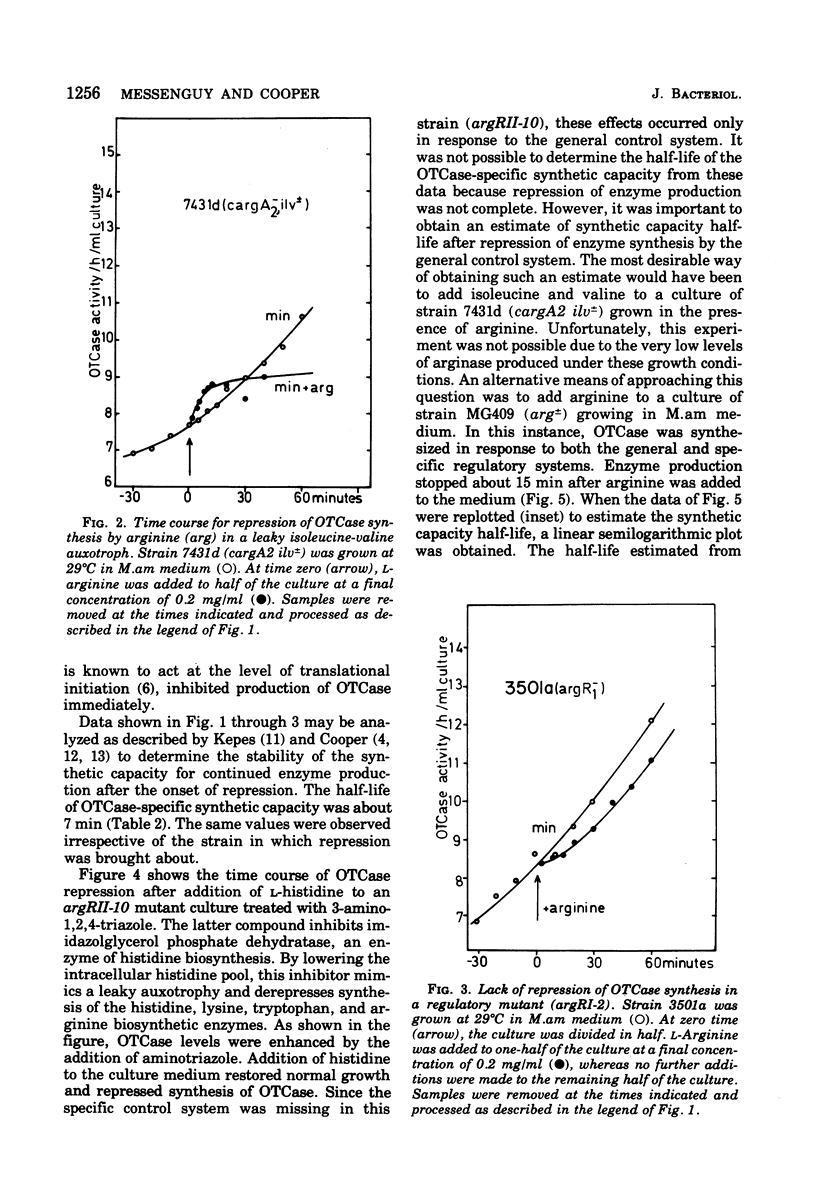
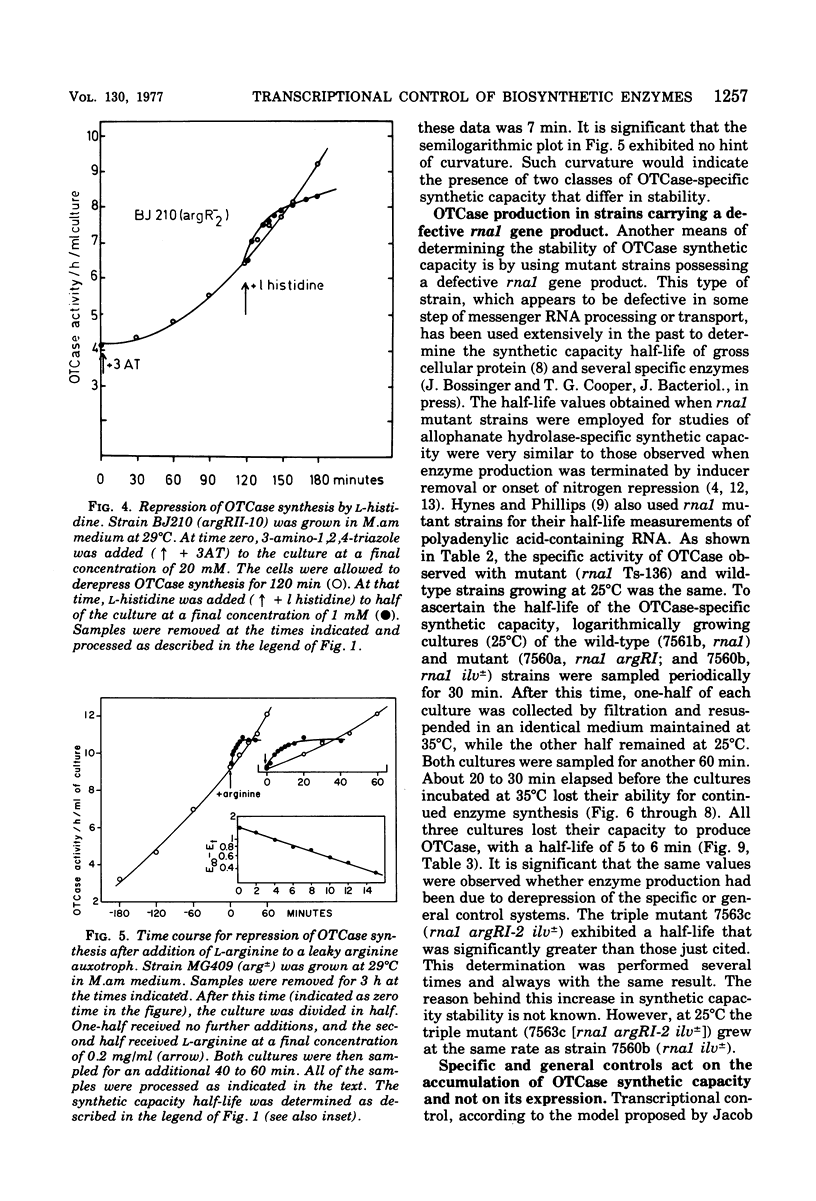
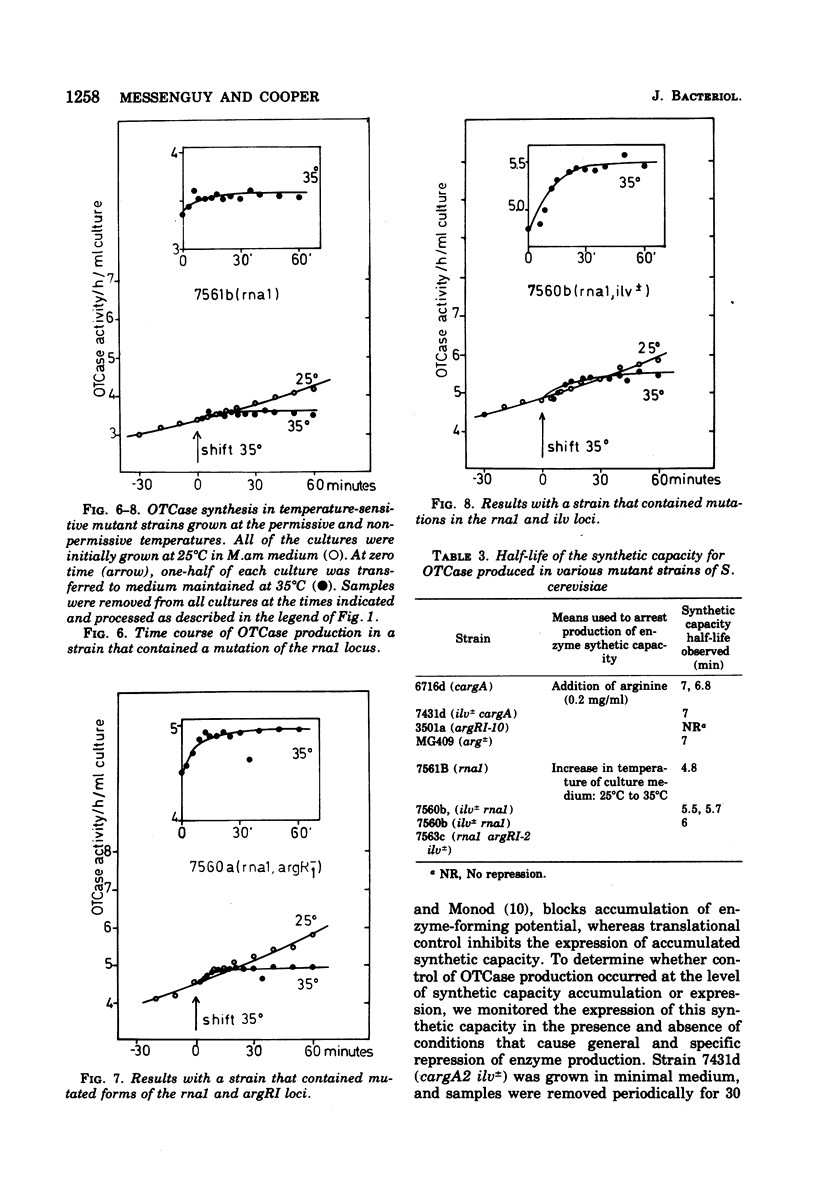
Ornithine carbamoyltransferase synthesis is subject to two major regulatory systems in Saccharomyces cerevisiae. One system is specific for the arginine biosynthetic enzymes, whereas the other appears to be general, acting on a variety of other amino acid pathways as well. We observed that the synthetic capacity for continued ornithine carbamoyltransferase synthesis had the same short half-life (ca. 5 to 7 min) whether repression of enzyme production was brought about by action of the specific or general control system. We present evidence suggesting that both control systems regulate accumulation or ornithine carbamoyltransferase-specific synthetic capacity, rather than modulating its expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Execution times of macromolecular synthetic processes involved in the induction of allophanate hydrolase at 15 degrees C. J Bacteriol. 1976 Oct;128(1):498–501. doi: 10.1128/jb.128.1.498-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Bossinger J. Selective inhibition of protein synthesis initiation in Saccharomyces cerevisiae by low concentrations of cycloheximide. J Biol Chem. 1976 Nov 25;251(22):7278–7280. [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Phillips S. L. Turnover of polyadenylate-containing ribonucleic acid in Saccharomyces cerevisiae. J Bacteriol. 1976 Feb;125(2):595–600. doi: 10.1128/jb.125.2.595-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Effects of inducer addition and removal upon the level of allophanate hydrolase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1100–1104. doi: 10.1016/s0006-291x(73)80008-7. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Delforge J. Role of transfer ribonucleic acids in the regulation of several biosyntheses in Saccharomyces cerevisiae. Eur J Biochem. 1976 Aug 16;67(2):335–339. doi: 10.1111/j.1432-1033.1976.tb10696.x. [DOI] [PubMed] [Google Scholar]
- Messenguy F. Regulation of arginine biosynthesis in Saccharomyces cerevisiae: isolation of a cis-dominant, constitutive mutant for ornithine carbamoyltransferase synthesis. J Bacteriol. 1976 Oct;128(1):49–55. doi: 10.1128/jb.128.1.49-55.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Wiame J. -M. The control of ornithinetranscarbamylase activity by arginase in Saccharomyces cerevisiae. FEBS Lett. 1969 Apr;3(1):47–49. doi: 10.1016/0014-5793(69)80093-1. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., McLaughlin C. S., Nierlich D. P. Half life of yeast messenger RNA. Nature. 1976 Mar 4;260(5546):70–72. doi: 10.1038/260070a0. [DOI] [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]


