Abstract
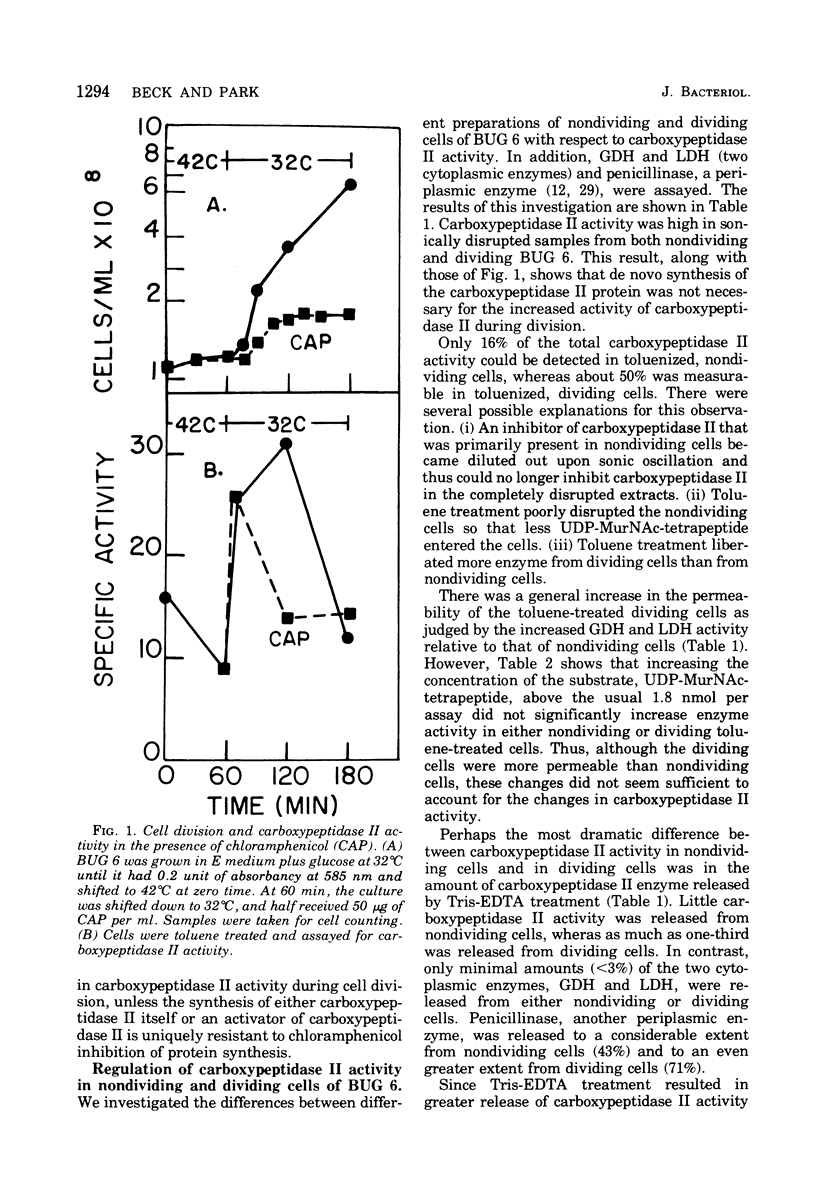
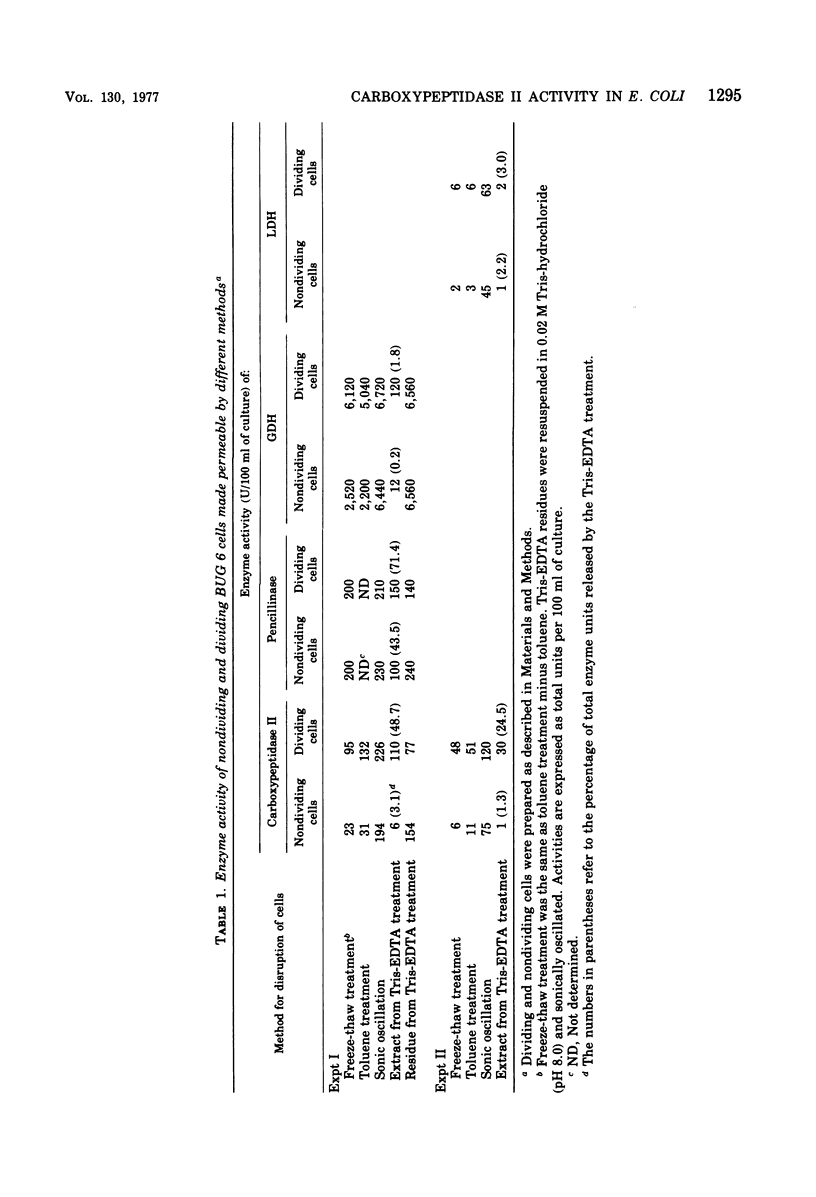
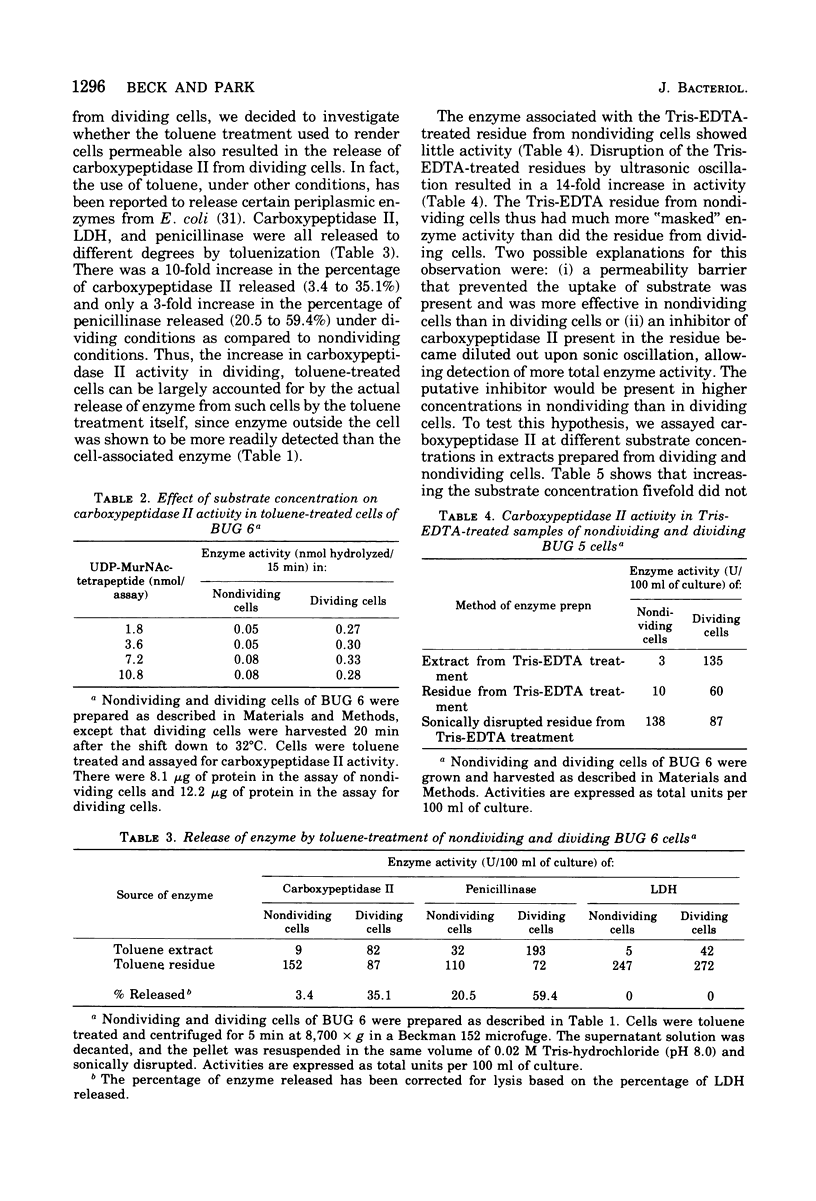
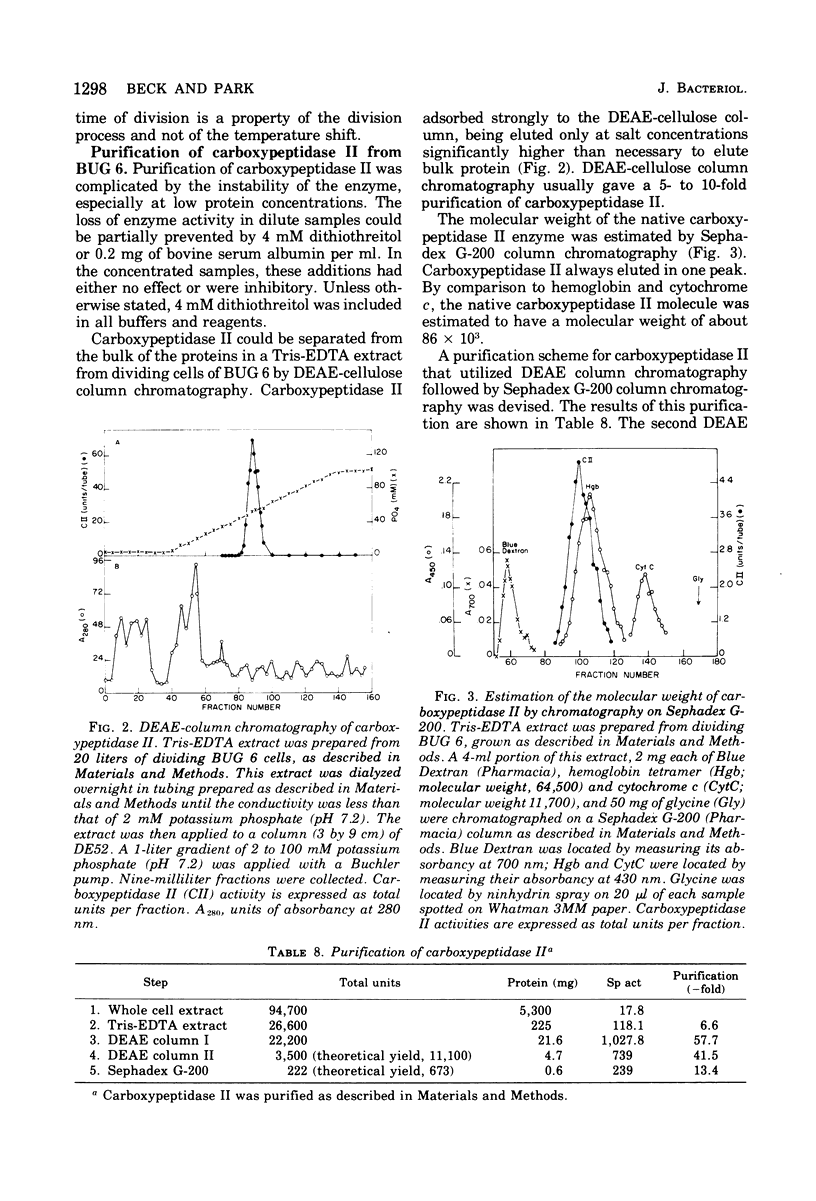
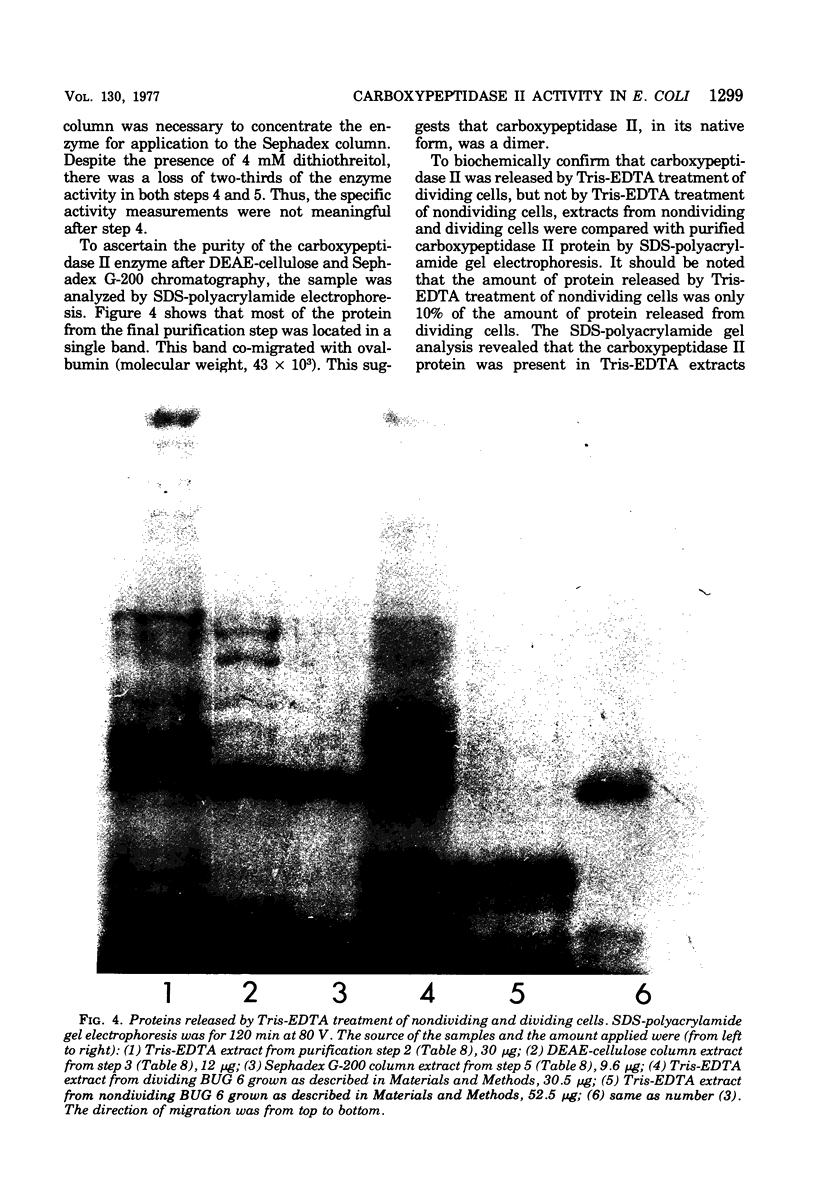
Diaminopimelyl-d-alanyl carboxypeptidase (carboxypeptidase II) is most active at the time of division, whether measured in toluene-treated cells of Escherichia coli K-12 strain D11-1, fractionated by size, or in toluene-treated cells of the temperature-sensitive division mutant, BUG 6 (B. D. Beck and J. T. Park, 1976). The present investigation has now shown that, under conditions that permit division, the increased carboxypeptidase II activity in toluenetreated cells of BUG 6 is probably not due to protein synthesis. Although dividing cells are more permeable than nondividing cells, permeability differences are not sufficient to account for the changes in carboxypeptidase II activity. Thus, in the toluene-treated nondividing cells, carboxypeptidase II is present, but its activity is masked, which suggests the presence of an inhibitor. Another striking difference between nondividing and dividing cells is that carboxypeptidase II is much more readily released from dividing cells by both tris(hydroxymethyl)aminomethane-ethylenediaminetetraacetic acid and toluene treatment. Carboxypeptidase II was partially purified and found to be an 86,000-molecular-weight protein consisting of two 43,000-molecular-weight polypeptides. Tris(hydroxymethyl)aminomethane-ethylenediaminetetraacetic acid treatment of nondividing cells releases less than 10% of the carboxypeptidase II and other periplasmic proteins that are releasable from dividing cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Wetzel B. K., Heppel L. A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a "minicell" strain of Escherichia coli. J Bacteriol. 1970 Oct;104(1):543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- James R., Gudas L. J. Cell cycle-specific incorporation of lipoprotein into the outer membrane of Escherichia coli. J Bacteriol. 1976 Jan;125(1):374–375. doi: 10.1128/jb.125.1.374-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Leive L. Release from Escherichia coli of a galactosyltransferase complex active in lipopolysaccharide synthesis. J Biol Chem. 1970 Feb 10;245(3):585–594. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shen B. H., Boos W. Regulation of the -methylgalactoside transport system and the galatose-binding protein by the cell cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1481–1485. doi: 10.1073/pnas.70.5.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G., Lazdunski A., Shapiro B. M. Interrelationship between membrane protein composition and deoxyribonucleic acid synthesis in Escherichia coli. Biochemistry. 1972 Apr 25;11(9):1573–1582. doi: 10.1021/bi00759a005. [DOI] [PubMed] [Google Scholar]
- Smith J. T., Wyatt J. M. Relation of R factor and chromosomal beta-lactamase with the periplasmic space. J Bacteriol. 1974 Mar;117(3):931–939. doi: 10.1128/jb.117.3.931-939.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Teuber M. Bedingungen für den Abbau von Ribonucleinsäure in Escherichia coli nach Zerstörung der cytoplasmatischen Membran durch Toluol. Arch Mikrobiol. 1966 Oct 19;55(1):31–45. [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weigand R. A., Rothfield L. I. Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol. 1976 Jan;125(1):340–345. doi: 10.1128/jb.125.1.340-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]