Abstract
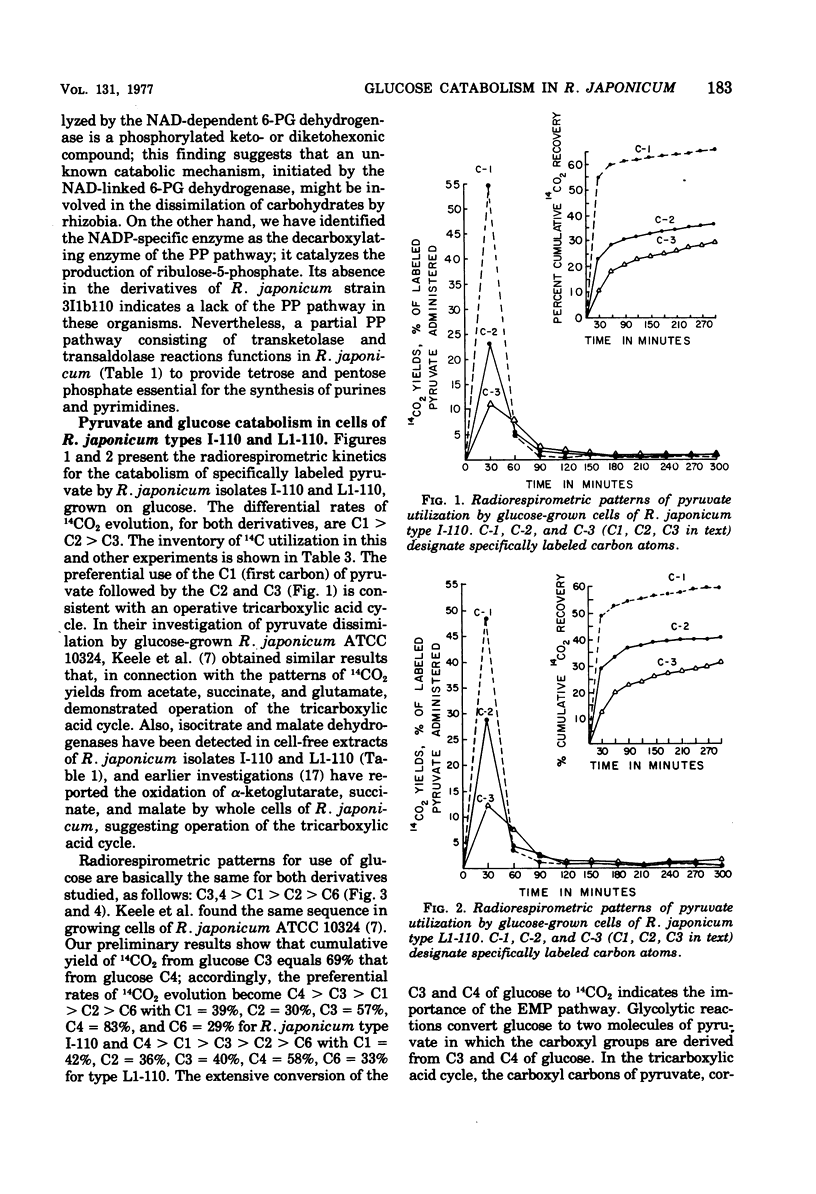
Radiorespirometric and enzymatic analyses reveal that glucose-grown cells of Rhizobium japonicum isolates I-110 and L1-110, both derivatives of R. japonicum strain 3I1b110, possess an active tricarboxylic acid cycle and metabolize glucose by simultaneous operation of the Embden-Meyerhof-Parnas and Entner-Doudoroff pathways. The hexose cycle may play a minor role in the dissimilation of glucose. Failure to detect the nicotinamide adenine dinucleotide phosphate-dependent decarboxylating 6-phosphogluconate dehydrogenase (EC 1.1.1.44) evidences absence of the pentose phosphate pathway. Transketolase and transaldolase reactions, however, enable R. japonicum to produce the precursors for purine and pyrimidine biosynthesis from fructose-6-phosphate and glyceraldehyde-3-phosphate. A constitutive nicotinamide adenine dinucleotide-linked 6-phosphogluconate dehydrogenase has been detected. The enzyme is stimulated by either mannitol or fuctose and might initiate a new catabolic pathway. R. japonicum isolate I-110, characterized by shorter generation times on glucose and greater nitrogen-fixing efficiency, oxidizes glucose more extensively than type L1-110 and utilizes preferentially the Embden-Meyerhof-Parnas pathway, whereas the Entner-Doudoroff pathway apparently predominates in type L1-110.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Dobrogosz W. J. Gluconate metabolism in Escherichia coli. J Bacteriol. 1967 Mar;93(3):941–949. doi: 10.1128/jb.93.3.941-949.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C. The bacteroids of the genus Rhizobium. Bacteriol Rev. 1962 Jun;26:119–141. [PMC free article] [PubMed] [Google Scholar]
- KATZNELSON H., ZAGALLO A. C. Metabolism of rhizobia in relation to effectiveness. Can J Microbiol. 1957 Oct;3(6):879–884. doi: 10.1139/m57-097. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Gluconate catabolism in Rhizobium japonicum. J Bacteriol. 1970 Mar;101(3):698–704. doi: 10.1128/jb.101.3.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Krieg N. R. Sugar catabolism in Aquaspirillum gracile. J Bacteriol. 1974 Sep;119(3):691–697. doi: 10.1128/jb.119.3.691-697.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland T. E., Kawasaki T., Lowenstein J. M. Comparative aspects of some bacterial dehydrogenases and transhydrogenases. J Bacteriol. 1966 Jan;91(1):236–244. doi: 10.1128/jb.91.1.236-244.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj H. D. Radiorespirometric studies of Leucothrix mucor. J Bacteriol. 1967 Sep;94(3):615–623. doi: 10.1128/jb.94.3.615-623.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STILL G. G., WANG C. H. GLUCOSE CATABOLISM IN AZOTOBACTER VINELANDII. Arch Biochem Biophys. 1964 Apr;105:126–132. doi: 10.1016/0003-9861(64)90243-7. [DOI] [PubMed] [Google Scholar]
- Vander Wyk J. C., Lessie T. G. Purification and characterization of the Pseudomonas multivorans glucose-6-phosphate dehydrogenase active with nicotinamide adenine dinucleotide. J Bacteriol. 1974 Dec;120(3):1033–1042. doi: 10.1128/jb.120.3.1033-1042.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


