Abstract
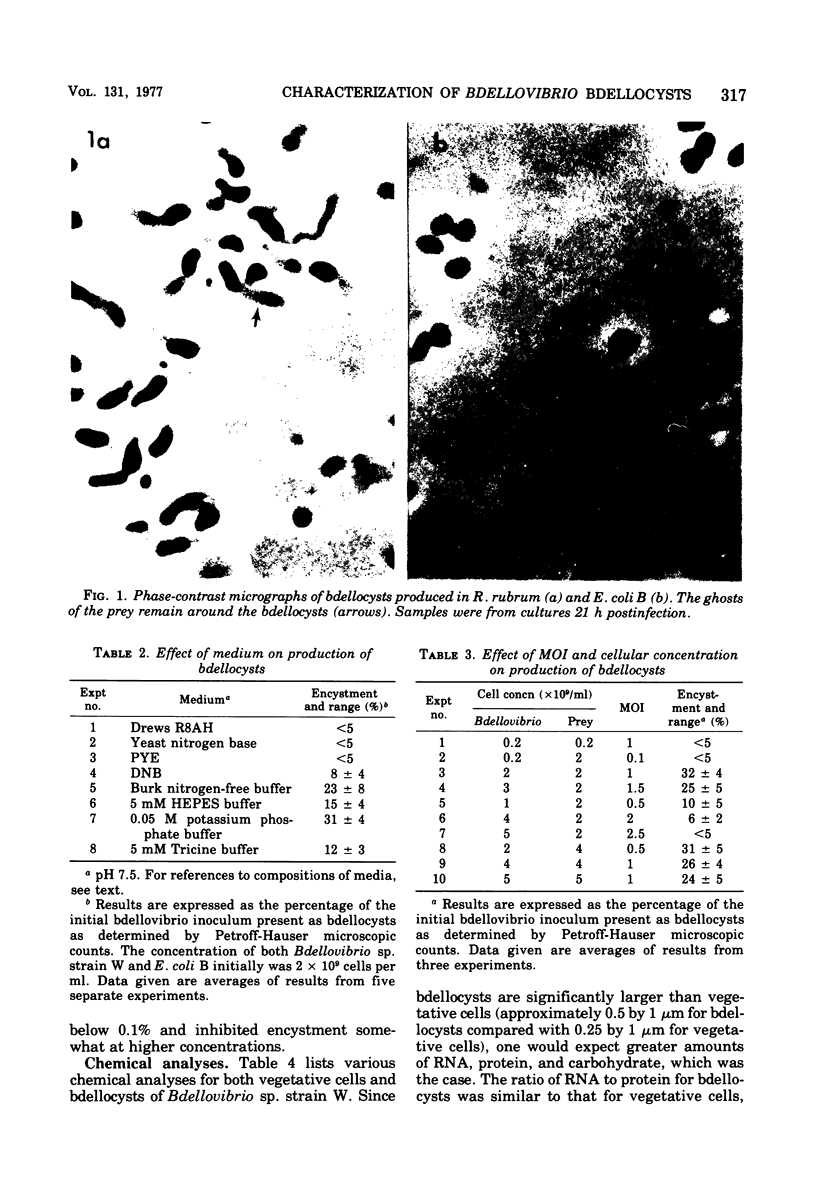
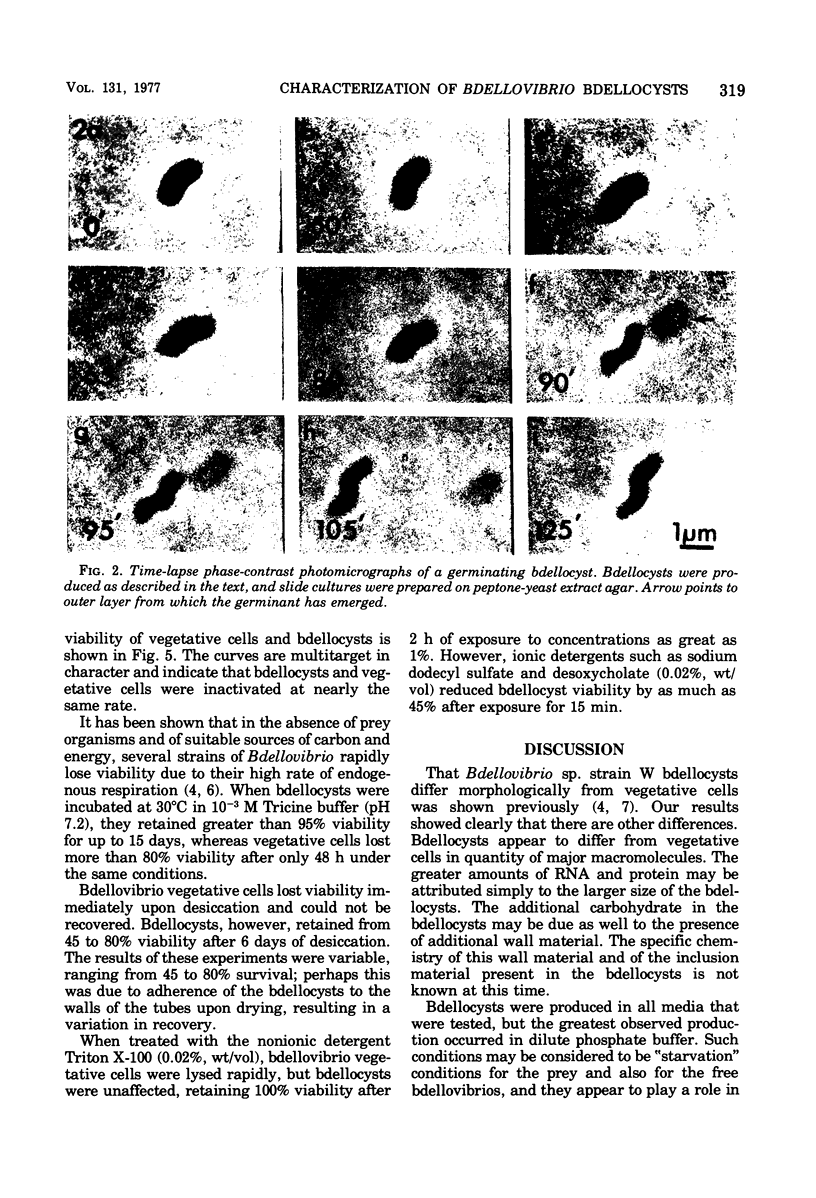
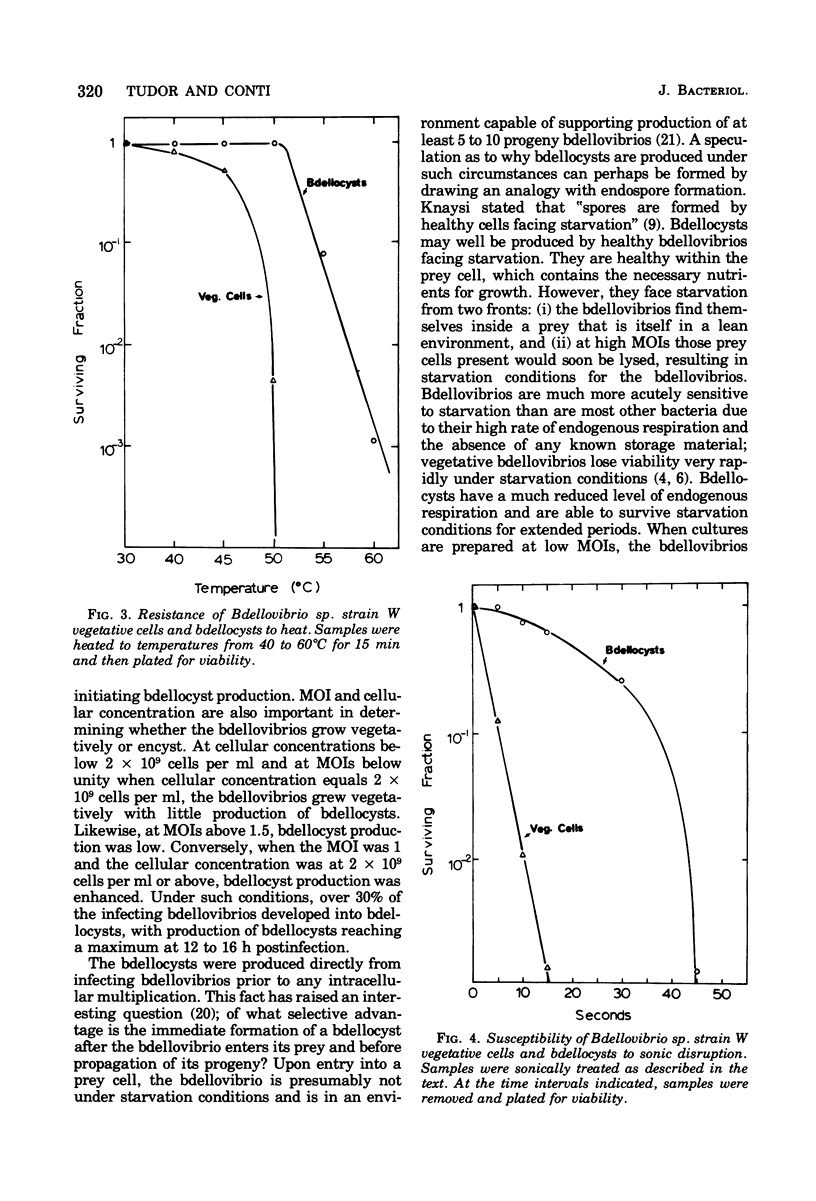
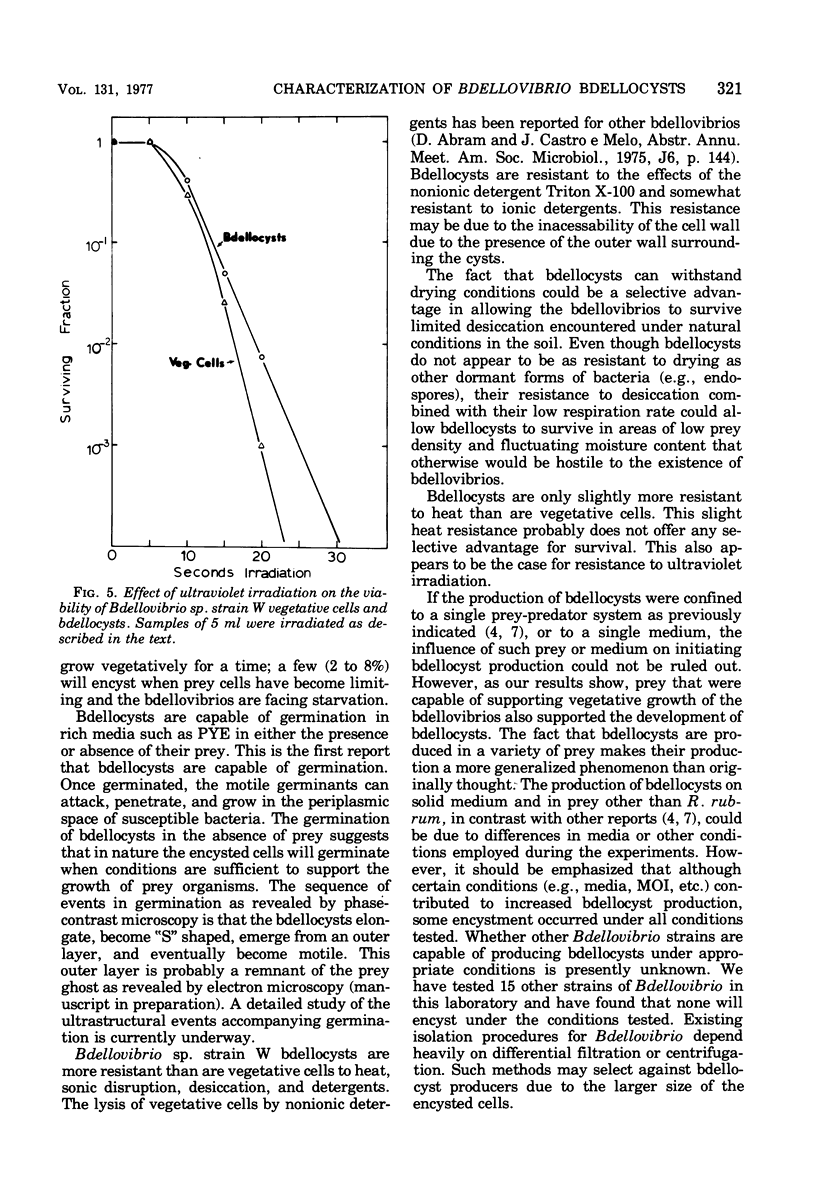
Bdellovibrio sp. strain W will infect and produce resting cells, termed bdellocysts, in a variety of gram-negative bacteria. Bdellocysts appeared to be produced only within susceptible prey and never in their absence. Optimum conditions for encystment included infection of stationary-phase prey cells in 0.05 M potassium phosphate buffer (pH 7.5) at concentrations of prey and bdellovibrios of 2 X 10(9) cells per ml with a multiplicity of infection of unity. Bdellocysts contained more deoxyribonucleic acid, ribonucleic acid, protein, and carbohydrate per cell than did vegetative cells. Poly-beta-hydroxybutyrate and dipicolinic acid were not detected. Bdellocysts were more resistant than vegetative cells to effects of elevated temperatures, sonic treatment, and desiccation. Bdellocysts remained viable for extended periods when incubated in the absence of prey, whereas vegetative cells lost viability rapidly under the same conditions. Their survival under starvation conditions may be due to the low rate of endogenous respiration by the bdellocysts. Bdellocysts are capable of germination in the presence or absence of prey cells in rich medium such as peptone-yeast extract.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althauser M., Samsonoff W. A., Anderson C., Conti S. F. Isolation and preliminary characterization of bacteriophages for Bdellovibrio bacteriovorus. J Virol. 1972 Sep;10(3):516–523. doi: 10.1128/jvi.10.3.516-523.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Drews G., Ladwig R. Wirtskreis und Infektionscyclus eines neu isolierten Bdellovibrio bacteriovorus-Stammes. Arch Mikrobiol. 1968 May 8;61(3):261–279. [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Ladwig R., Moor H. The fine structure of "resting bodies" of Bdellovibrio sp. strain W developed in Rhodospirillum rubrum. Can J Microbiol. 1972 Jan;18(1):87–92. doi: 10.1139/m72-014. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Knaysi G. THE ENDOSPORE OF BACTERIA. Bacteriol Rev. 1948 Mar;12(1):19–77. doi: 10.1128/br.12.1.19-77.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Kinetics of deoxyribonucleic acid destruction and synthesis during growth of Bdellovibrio bacteriovorus strain 109D on pseudomonas putida and escherichia coli. J Bacteriol. 1972 Sep;111(3):664–673. doi: 10.1128/jb.111.3.664-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Shilo M. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Apr;102(1):149–160. doi: 10.1128/jb.102.1.149-160.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCOLOFSKY M. D., WYSS O. Resistance of the Azotobacter cyst. J Bacteriol. 1962 Jul;84:119–124. doi: 10.1128/jb.84.1.119-124.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J Bacteriol. 1969 Feb;97(2):912–923. doi: 10.1128/jb.97.2.912-923.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P., Mandel M. Deoxyribonucleic acid characterization of Bdellovibrios. J Bacteriol. 1969 Nov;100(2):786–790. doi: 10.1128/jb.100.2.786-790.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M. Morphological and physiological aspects of the interaction of Bdellovibrio with host bacteria. Curr Top Microbiol Immunol. 1969;50:174–204. doi: 10.1007/978-3-642-46169-9_6. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Seidler R. J. The Bdellovibros. Annu Rev Microbiol. 1971;25:649–678. doi: 10.1146/annurev.mi.25.100171.003245. [DOI] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]