Abstract
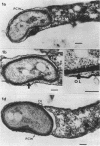
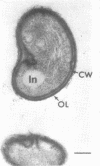
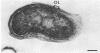
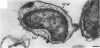
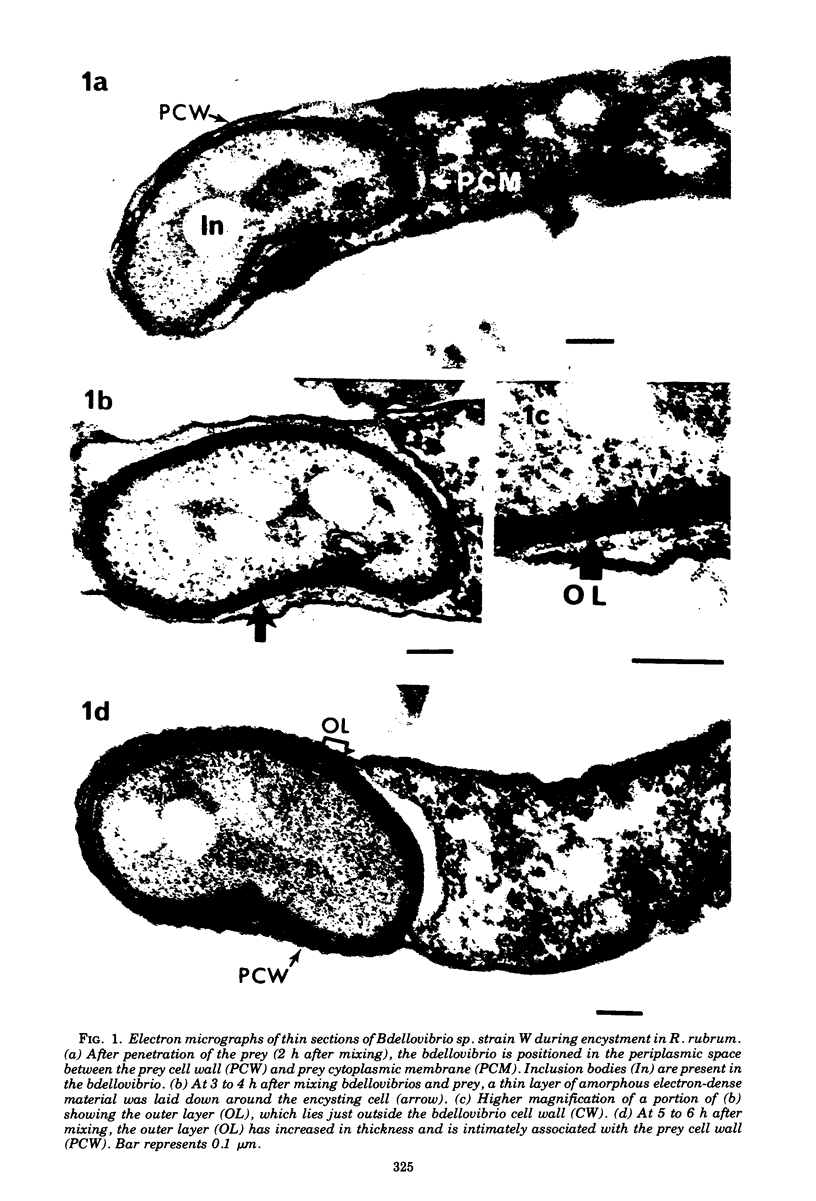
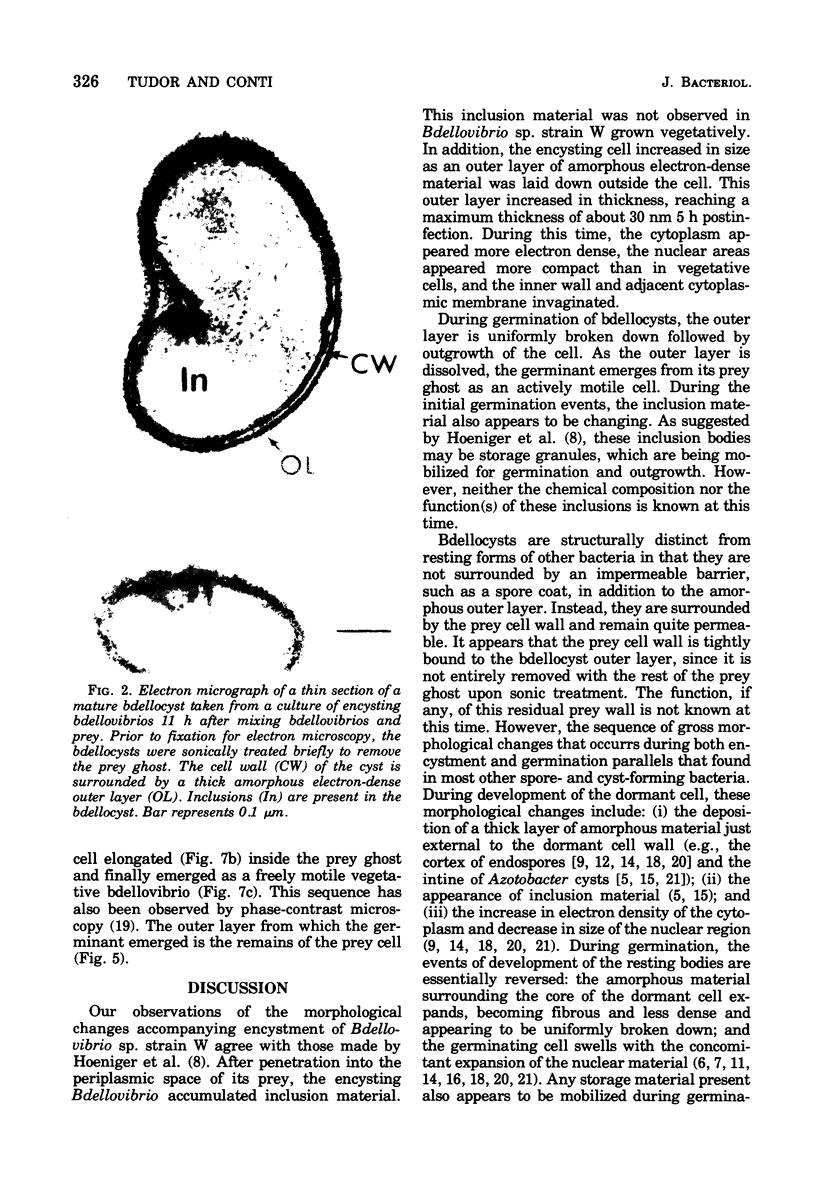
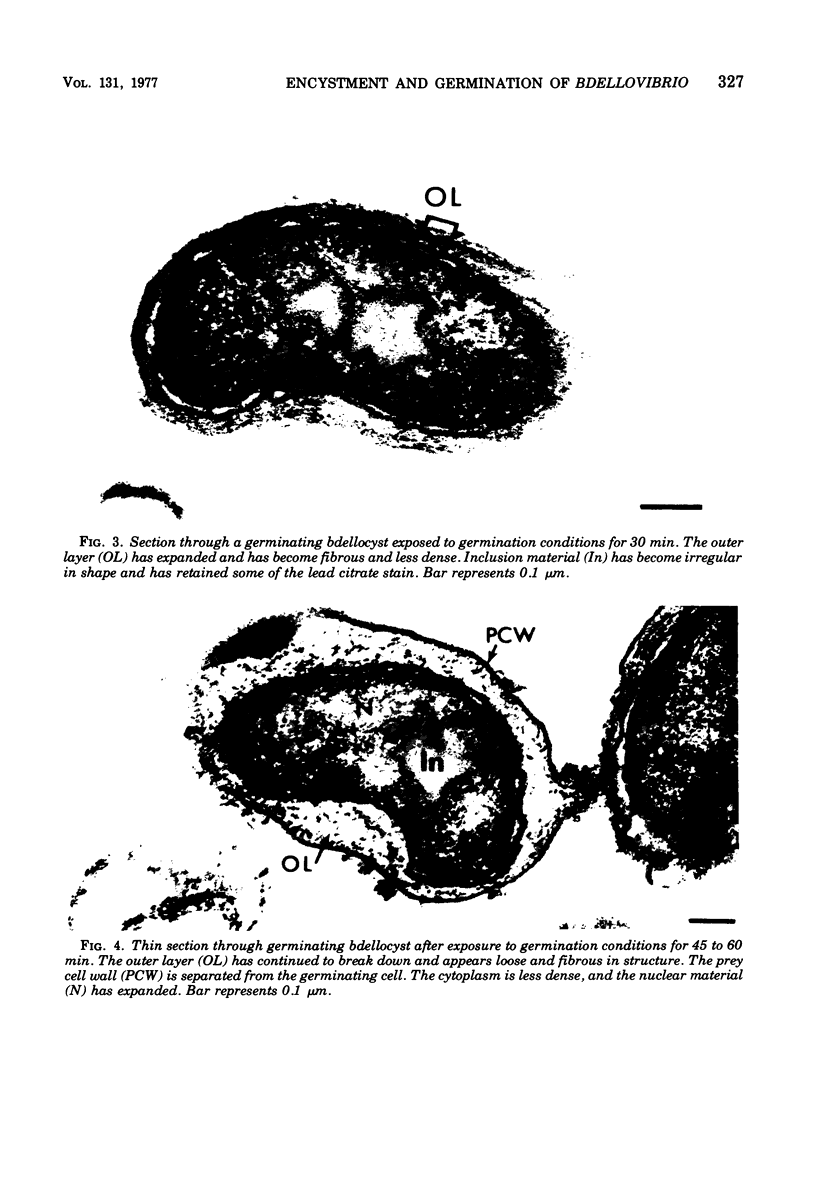
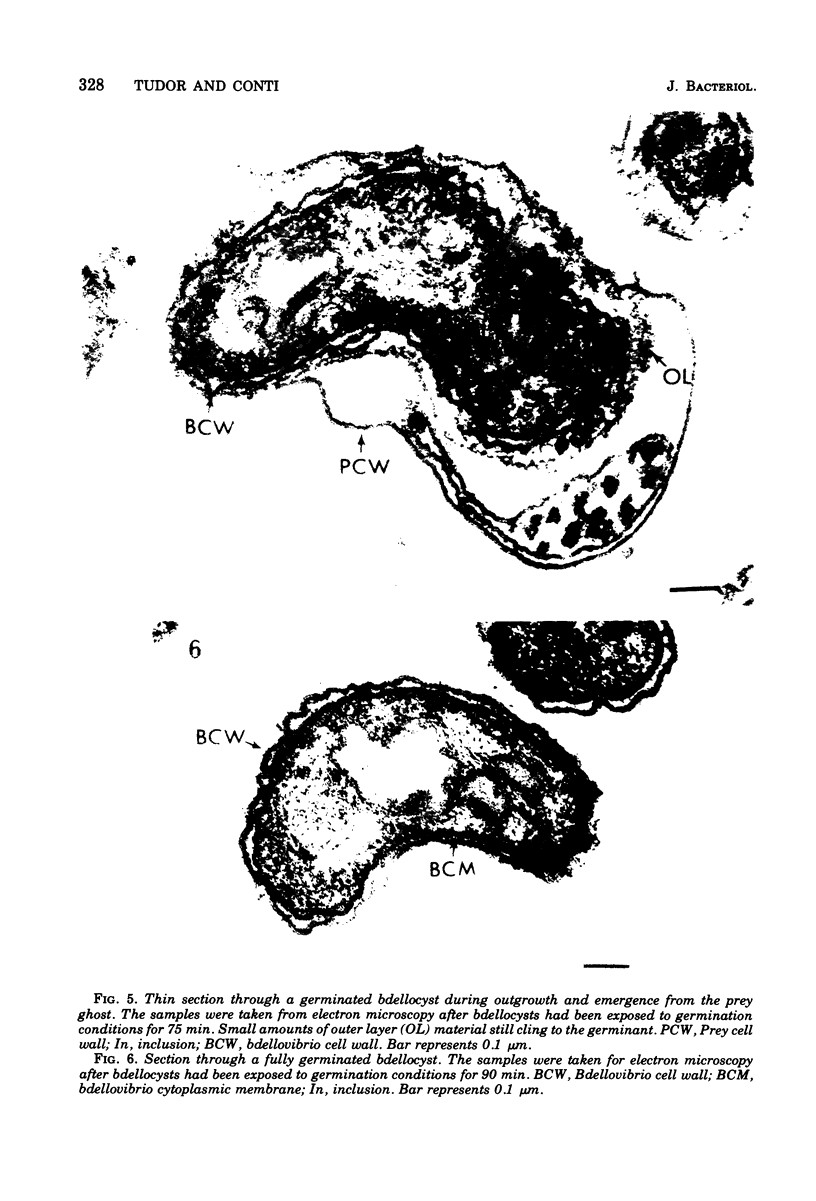
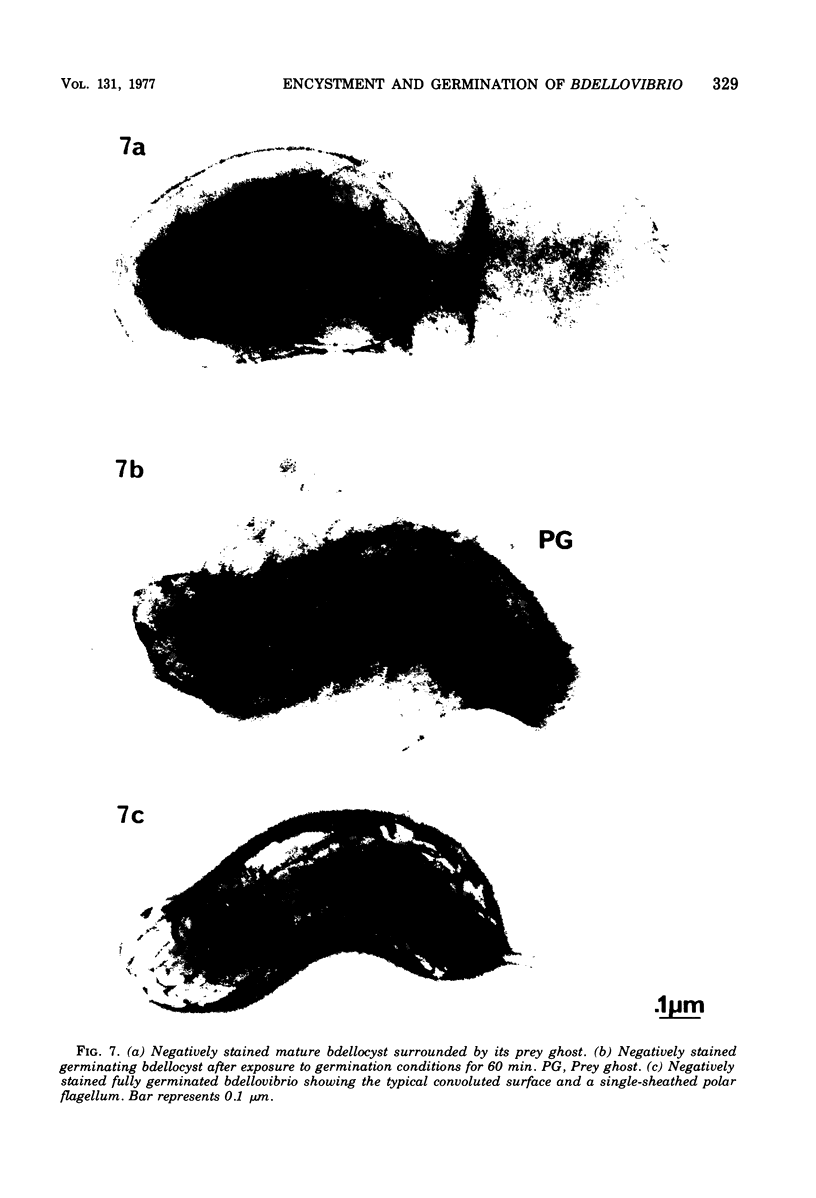
Under proper conditions, Bdellovibrio sp. strain W cells develop into bdellocysts in appropriate prey bacteria. After attachment and penetration of the prey cell, the encysting bdellovibrio began to accumulate inclusion material and increase in size, and was surrounded by an outer layer of amorphous electrondense material. The cytoplasm of the encysting cell appeared more electron dense, and nuclear areas appeared more compact. During germination of bdellocysts, the outer wall was uniformly broken down the inclusion material changed shape and affinity for the heavy metal stain, and the nuclear areas expanded. As the outer wall was dissolved, outgrowth began with the elongation of the germinant as it emerged from the prey ghost as an actively motile cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althauser M., Samsonoff W. A., Anderson C., Conti S. F. Isolation and preliminary characterization of bacteriophages for Bdellovibrio bacteriovorus. J Virol. 1972 Sep;10(3):516–523. doi: 10.1128/jvi.10.3.516-523.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Drews G., Ladwig R. Wirtskreis und Infektionscyclus eines neu isolierten Bdellovibrio bacteriovorus-Stammes. Arch Mikrobiol. 1968 May 8;61(3):261–279. [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J Bacteriol. 1968 Oct;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Denny C. F., Hashimoto T., Conti S. F. Facultatively parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Mar;101(3):989–996. doi: 10.1128/jb.101.3.989-996.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins V. M., Sadoff H. L. Morphogenesis of cysts in Azotobacter vinelandii. J Bacteriol. 1970 Oct;104(1):492–498. doi: 10.1128/jb.104.1.492-498.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger J. F., Headley C. L. Cytology of spore germination in Clostridium pectinovorum. J Bacteriol. 1968 Nov;96(5):1835–1847. doi: 10.1128/jb.96.5.1835-1847.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger J. F., Headley C. L. Ultrastructural aspects of spore germination and outgrowth in Clostridium sporogenes. Can J Microbiol. 1969 Sep;15(9):1061–1065. doi: 10.1139/m69-189. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Ladwig R., Moor H. The fine structure of "resting bodies" of Bdellovibrio sp. strain W developed in Rhodospirillum rubrum. Can J Microbiol. 1972 Jan;18(1):87–92. doi: 10.1139/m72-014. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Stuart P. F., Holt S. C. Cytology of spore formation in Clostridium perfringens. J Bacteriol. 1968 Nov;96(5):1818–1834. doi: 10.1128/jb.96.5.1818-1834.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonoff W. A., Hashimoto T., Conti S. F. Ultrastructural changes associated with germination and outgrowth of an appendage-bearing clostridial spore. J Bacteriol. 1970 Mar;101(3):1038–1045. doi: 10.1128/jb.101.3.1038-1045.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J Bacteriol. 1969 Feb;97(2):912–923. doi: 10.1128/jb.97.2.912-923.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAGI A., KAWATA T., YAMAMOTO S. Electron microscope studies on ultrathin sections of spores of the Clostridium group, with special reference to the sporulation and germination process. J Bacteriol. 1960 Jul;80:37–46. doi: 10.1128/jb.80.1.37-46.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor J. J., Conti S. F. Characterization of bdellocysts of Bdellovibrio sp. J Bacteriol. 1977 Jul;131(1):314–322. doi: 10.1128/jb.131.1.314-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYSS O., NEUMNN M. G., SOCOLOFSKY M. D. Development and germination of the Azotobacter cyst. J Biophys Biochem Cytol. 1961 Aug;10:555–565. doi: 10.1083/jcb.10.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D. Symposium on bacterial spores: I. Cytology of spore formation and germination. J Appl Bacteriol. 1970 Mar;33(1):1–12. doi: 10.1111/j.1365-2672.1970.tb05229.x. [DOI] [PubMed] [Google Scholar]