Abstract
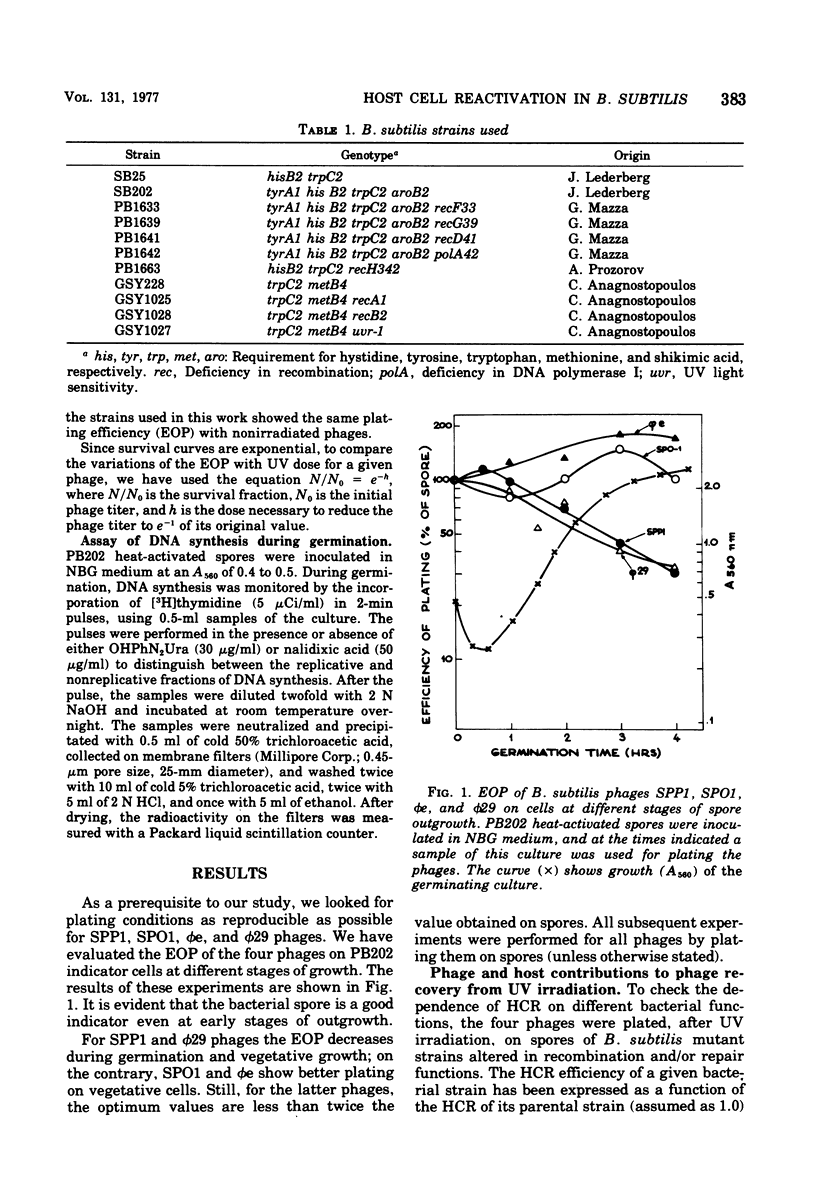
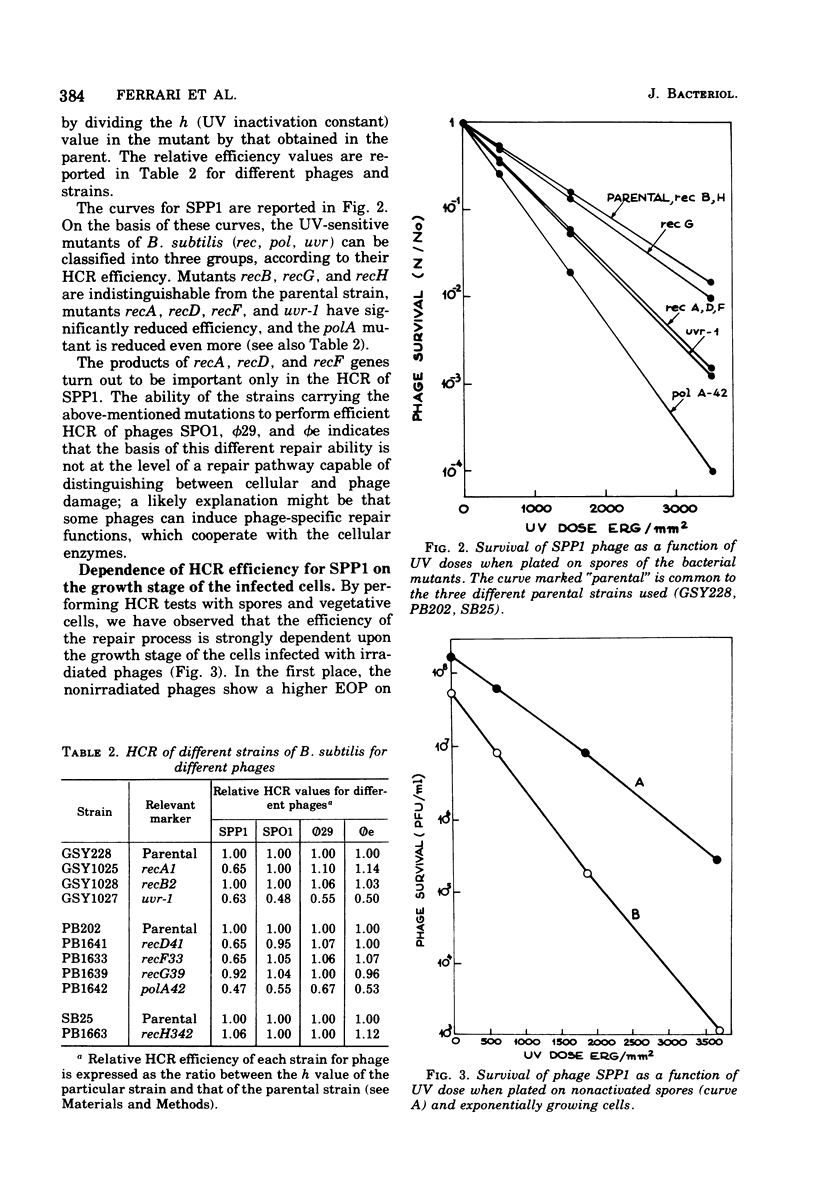
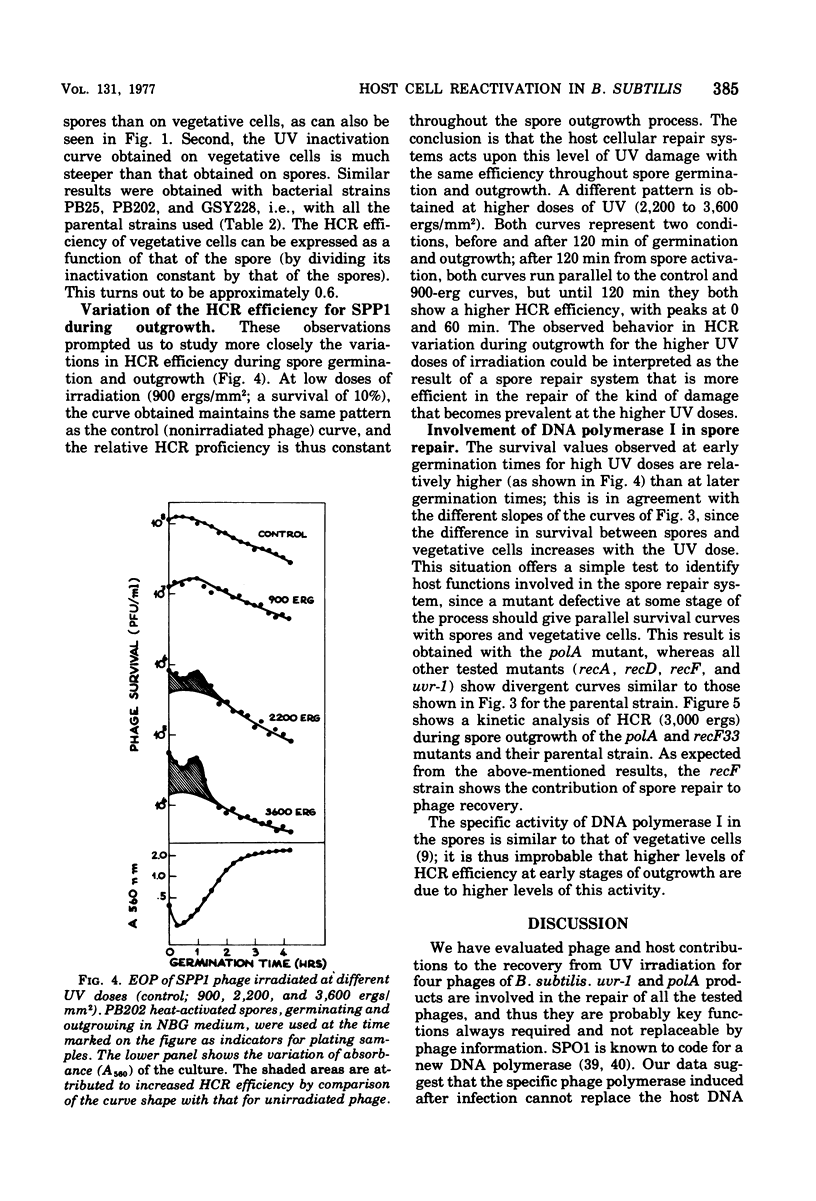
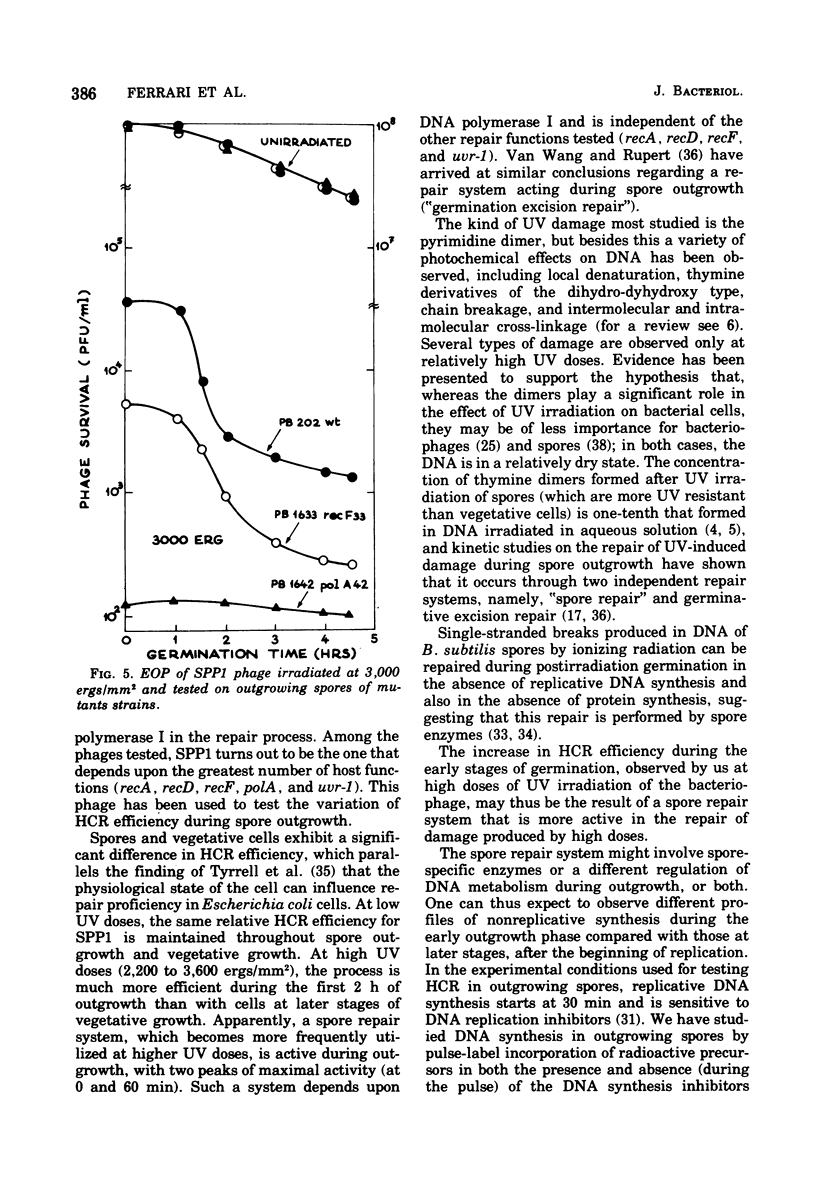
Host cell reactivation of ultraviolet-irradiated phage can be used as a probe of the bacterial repair system and to determine phage and cellular contributions to the repair process. Using the Bacillus subtilis phages SPP1, SP01, phie, and phi29, we found that the uvr-1 and polA functions are involved in the host cell reactivation of the four phages. SPP1 was the only phage whose reactivation was also decreased in recA, recD, and recF mutant cells. We studied variations of host cell reactivation for SPP1 during spore outgrowth; at high ultraviolet doses the activity of a spore repair system requiring deoxyribonucleic acid polymerase I became evident. The spore repair system was completely replaced by the vegetative one by 120 min of outgrowth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Stafford R. S. The ultraviolet photochemistry and photobiology of vegetative cells and spores of Bacillus megaterium. Biophys J. 1968 Jan;8(1):17–28. doi: 10.1016/S0006-3495(68)86471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney C. O. Ultraviolet light effects on the bacterial cell. Curr Top Microbiol Immunol. 1968;46:116–175. doi: 10.1007/978-3-642-46121-7_4. [DOI] [PubMed] [Google Scholar]
- ELLISON S. A., FEINER R. R., HILL R. F. A host effect on bacteriophage survival after ultraviolet irradiation. Virology. 1960 May;11:294–296. doi: 10.1016/0042-6822(60)90069-6. [DOI] [PubMed] [Google Scholar]
- Elder R. L., Beers R. F. Nonphotoreactivating Repair of Ultraviolet Light-Damaged Transforming Deoxyribonucleic Acid by Micrococcus lysodeikticus Extracts. J Bacteriol. 1965 Sep;90(3):681–686. doi: 10.1128/jb.90.3.681-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaschi A., Kornberg A. Biochemical studies of bacterial sporulation. II. Deoxy- ribonucleic acid polymerase in spores of Bacillus subtilis. J Biol Chem. 1966 Apr 10;241(7):1478–1482. [PubMed] [Google Scholar]
- Ganesan A. T., Yehle C. O., Yu C. C. DNA replication in a polymerase I deficient mutant and the identification of DNA polymerases II and 3 in Bacillus subtilis. Biochem Biophys Res Commun. 1973 Jan 4;50(1):155–163. doi: 10.1016/0006-291x(73)91077-2. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Hill T. C., Goulian M., Strauss B. S., Cozzarelli N. R. Altered deoxyribonucleic acid polymerase activity in a methyl methanesulfonate-sensitive mutant of Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):364–374. doi: 10.1128/jb.108.1.364-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARM W. On the relationship between host-cell reactivation and UV-reactivation in UV-inactivated phages. Z Vererbungsl. 1963;94:67–79. doi: 10.1007/BF00895157. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., BOYCE R. P., SIMSON E., THERIOT L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Mazza G., Fortunato A., Ferrari E., Canosi U., Falaschi A., Polsinelli M. Genetic and enzymic studies on the recombination process in Bacillus subtilis. Mol Gen Genet. 1975;136(1):9–30. doi: 10.1007/BF00275445. [DOI] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Dark repair of DNA containing "spore photoproduct" in Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Effects of DNA-polymerase-defective and recombination-deficient mutations on the ultraviolet sensitivity of Bacillus subtilis spores. Mutat Res. 1975 Feb;27(2):157–169. doi: 10.1016/0027-5107(75)90075-5. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- ROERSCH A., VAN DER KAM P. C., ADEMA J. DARK REACTIVATION OF ULTRAVIOLET IRRADIATED BACTERIOPHAGE DEOXYRIBONUCLEIC ACID IN VITRO. Biochim Biophys Acta. 1964 Feb 17;80:346–348. [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., COHEN J. A. The gene-controlled radiation sensitivity in Escherichia coli. Biochim Biophys Acta. 1963 Feb 26;68:263–270. doi: 10.1016/0006-3002(63)90141-0. [DOI] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- Riklis E. Studies on mechanism of repair of ultraviolet-irradiated viral and bacterial DNAin vivo and in vitro. Can J Biochem. 1965 Jul;43(7):1207–1219. doi: 10.1139/o65-132. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- SAUERBIER W. Evidence for a nonrecombinational mechanism of host cell reactivation of phage. Virology. 1962 Apr;16:398–404. doi: 10.1016/0042-6822(62)90219-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano H., Tanooka H., Kadota H. Germination-induced repair of single-strand breaks of DNA in irradiated Bacillus subtilis spores. Biochem Biophys Res Commun. 1969 Sep 24;37(1):66–71. doi: 10.1016/0006-291x(69)90881-x. [DOI] [PubMed] [Google Scholar]
- Terano H., Tanooka H., Kadota H. Repair of radiation damage to deoxyribonucleic acid in germinating spores of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):925–930. doi: 10.1128/jb.106.3.925-930.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell R. M., Moss S. H., Davies D. J. The variation in UV sensitivity of four K12 strains of Escherichia coli as a function of their stage of growth. Mutat Res. 1972 Sep;16(7):1–12. doi: 10.1016/0027-5107(72)90058-9. [DOI] [PubMed] [Google Scholar]
- Villani G., Canosi U., Fortunato A., Mazza G., Polsinelli M., Falaschi A. Properties of a Bacillus subtilis strain lacking DNA polymerase I. Nucleic Acids Res. 1974 Mar;1(3):461–477. doi: 10.1093/nar/1.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Rupert C. S. Transitory germinative excision repair in Bacillus subtilis. J Bacteriol. 1977 Mar;129(3):1313–1319. doi: 10.1128/jb.129.3.1313-1319.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SP01-infected Bacillus subtilis. II. Purification and catalytic properties of a deoxyribonucleic acid polymerase induced after infection. J Biol Chem. 1973 Nov 10;248(21):7456–7463. [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SPO1-infected Bacillus subtilis. I. Bacteriophage deoxyribonucleic acid synthesis and fate of host deoxyribonucleic acid in normal and polymerase-deficient strains. J Virol. 1972 Feb;9(2):263–272. doi: 10.1128/jvi.9.2.263-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S., Reddy T. K. Induction of mutations by ultraviolet irradiation of spores of Bacillus subtilis. Radiat Res. 1967 May;31(1):112–120. [PubMed] [Google Scholar]


