Abstract
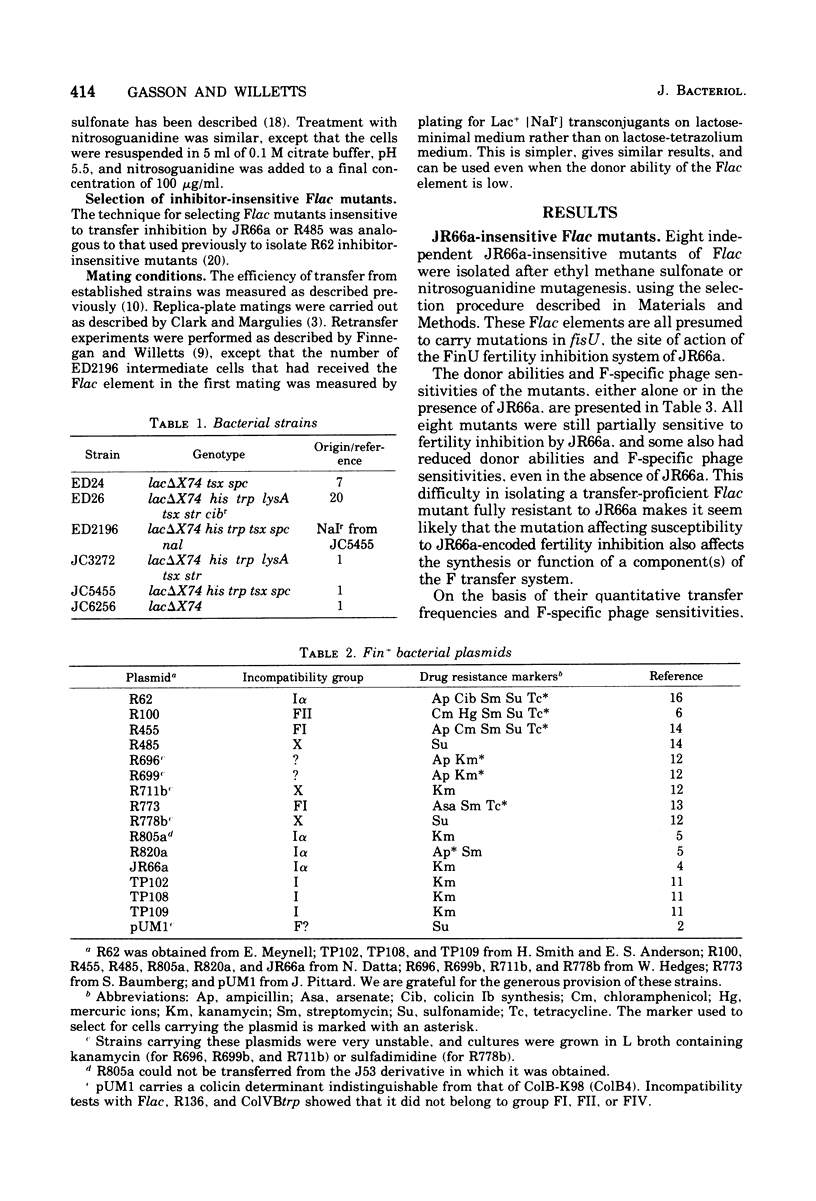
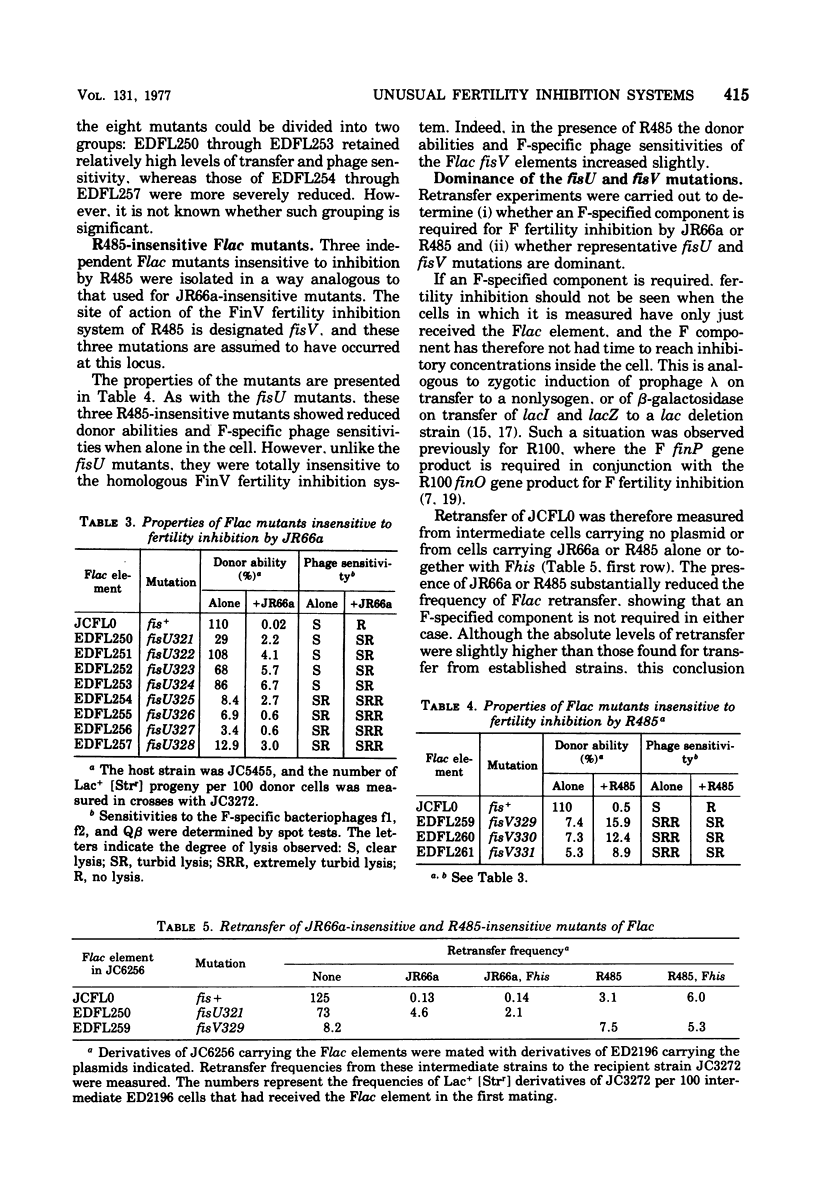
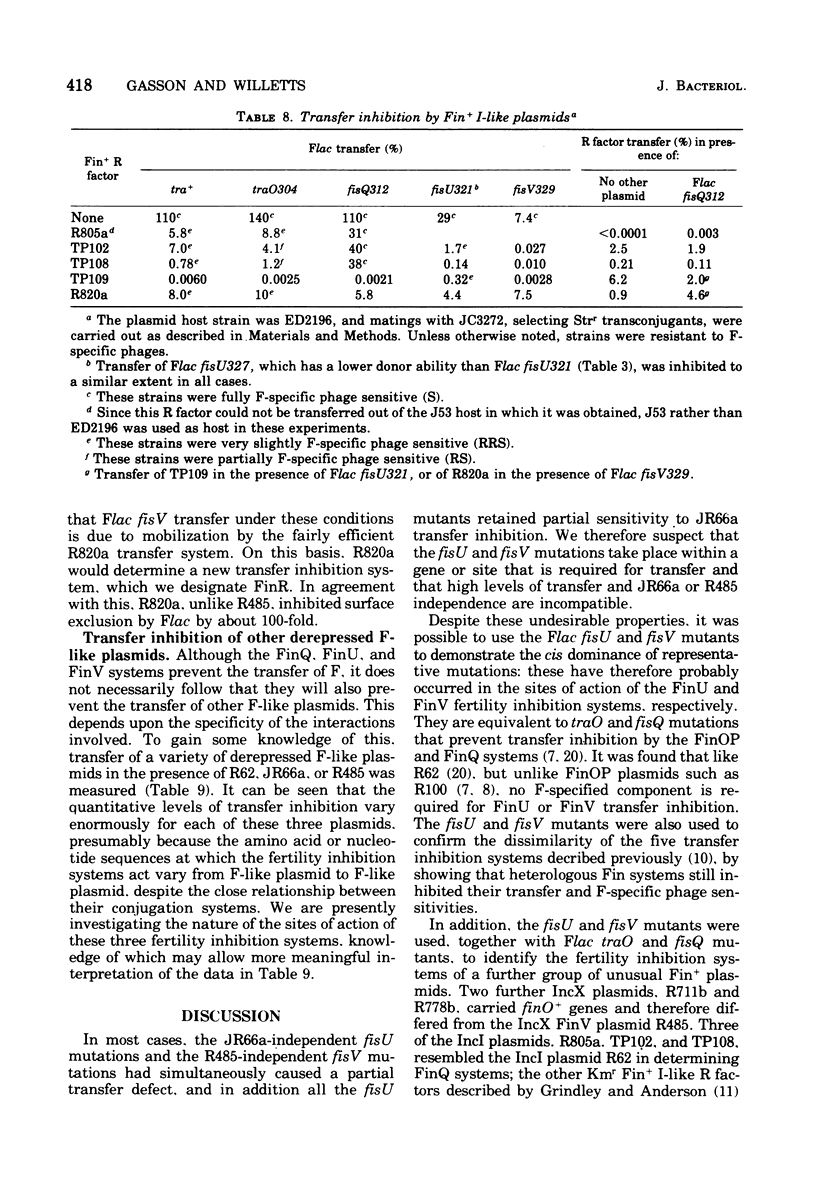
Flac mutants insensitive to transfer inhibition by R factors. JR66a and R485 were isolated and characterized. Representative mutations were cis dominant and are therefore presumed to be at the sites of action, fisU and fisV, respectively, of the FinU and FinV transfer inhibition systems encoded by JR66a and R485. The mutants were used to confirm that the FinU and FinV fertility inhibition systems are different from each other and from the FinOP, FinQ, and FinW systems of R100, R62, and R455, respectively. Together with traO and fisQ mutants of Flac, the new mutants were also used to investigate the nature of the F fertility inhibition systems encoded by a further group of "unusual" Fin+ plasmids. Of these, two incompatibility group X plasmids were found to carry finO+ genes, and of five incompatibility group I plasmids, three encoded FinQ systems, one the FinU system, and one a new system (FinR). Transfer of a variety of derepressed F-like plasmids was inhibited by the FinQ, FinU, and FinV systems, but a quantitatively very different levels; this emphasizes the differences as well as the similarities between the conjugation systems of F-like plasmids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. An I pilus-determining R factor with anomalous compatibility properties, mobilizing a gentamicin-resistance plasmid. J Gen Microbiol. 1973 Jul;77(1):11–17. doi: 10.1099/00221287-77-1-11. [DOI] [PubMed] [Google Scholar]
- Datta N., Olarte J. R factors in strains of Salmonella typhi and Shigella dysenteriae 1 isolated during epidemics in Mexico: classification by compatibility. Antimicrob Agents Chemother. 1974 Mar;5(3):310–317. doi: 10.1128/aac.5.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. B., Pittard J. Genetic and biophysical study of R plasmids conferring sulfonamide resistance in Shigella strains isolated in 1952 and 1956. J Bacteriol. 1974 Dec;120(3):1186–1195. doi: 10.1128/jb.120.3.1186-1195.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The nature of the transfer inhibitor of several F-like plasmids. Mol Gen Genet. 1972;119(1):57–66. doi: 10.1007/BF00270444. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Willetts N. S. Five control systems preventing transfer of Escherichia coli K-12 sex factor F. J Bacteriol. 1975 May;122(2):518–525. doi: 10.1128/jb.122.2.518-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Coetzee J. N., Dennison S. R factors from Proteus morganii. J Gen Microbiol. 1973 Aug;77(2):249–259. doi: 10.1099/00221287-77-2-249. [DOI] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. doi: 10.1099/00221287-81-1-171. [DOI] [PubMed] [Google Scholar]
- Meynell E. Pseudo-fi + I-like sex factors, R62(I), selective for increased pilus synthesis. J Bacteriol. 1973 Jan;113(1):502–503. doi: 10.1128/jb.113.1.502-503.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Characterization of the F transfer cistron, traL. Genet Res. 1973 Apr;21(2):205–213. doi: 10.1017/s0016672300013379. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Paranchych W. Inhibition of Flac transfer by the fin+ I-like plasmid R62. J Bacteriol. 1974 Oct;120(1):101–105. doi: 10.1128/jb.120.1.101-105.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. The kinetics of inhibition of Flac transfer by R100 in E. coli. Mol Gen Genet. 1974 Mar 14;129(2):123–130. doi: 10.1007/BF00268626. [DOI] [PubMed] [Google Scholar]


