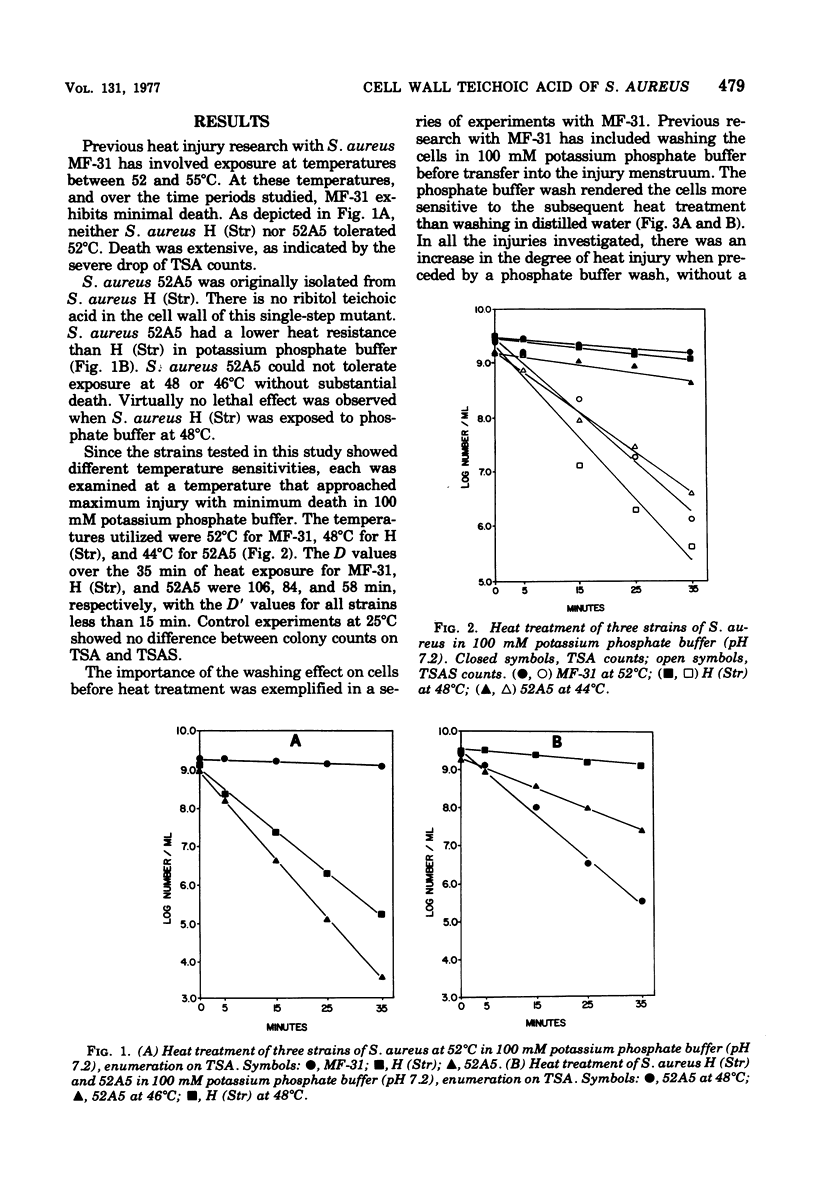
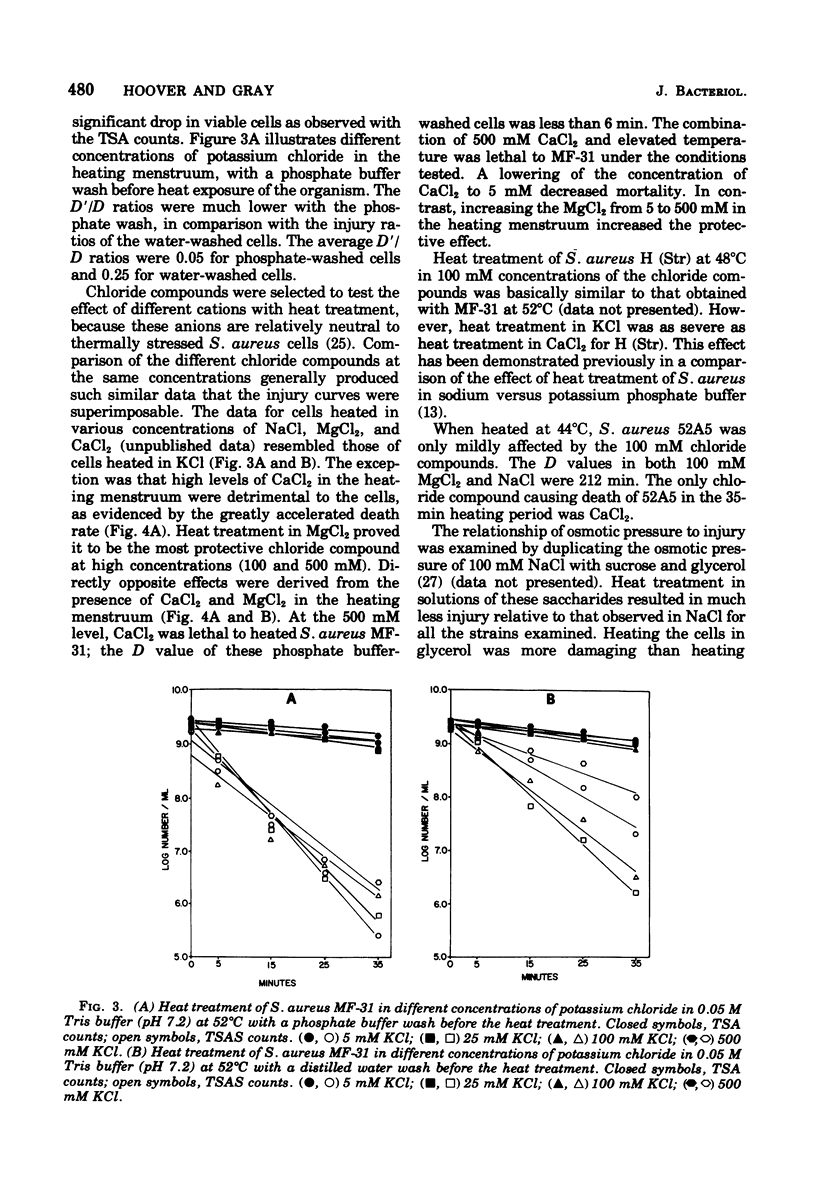
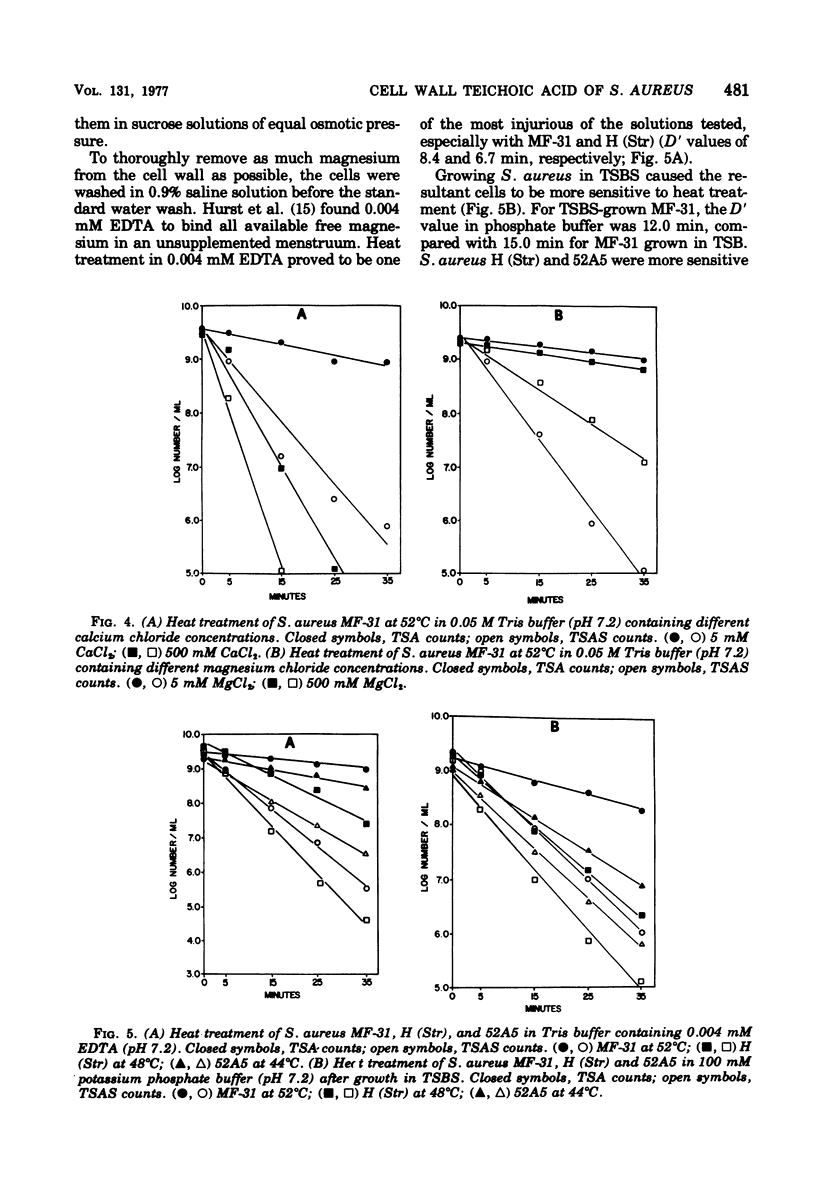
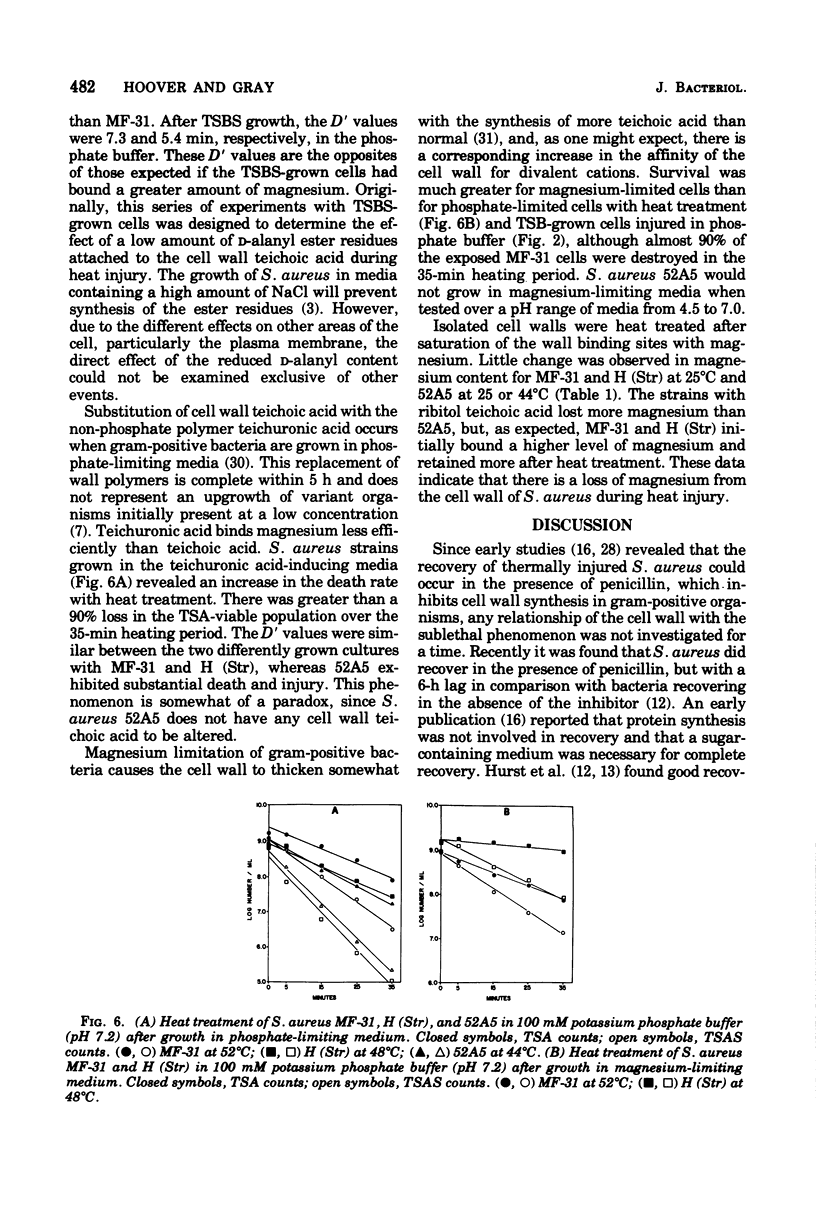
Abstract
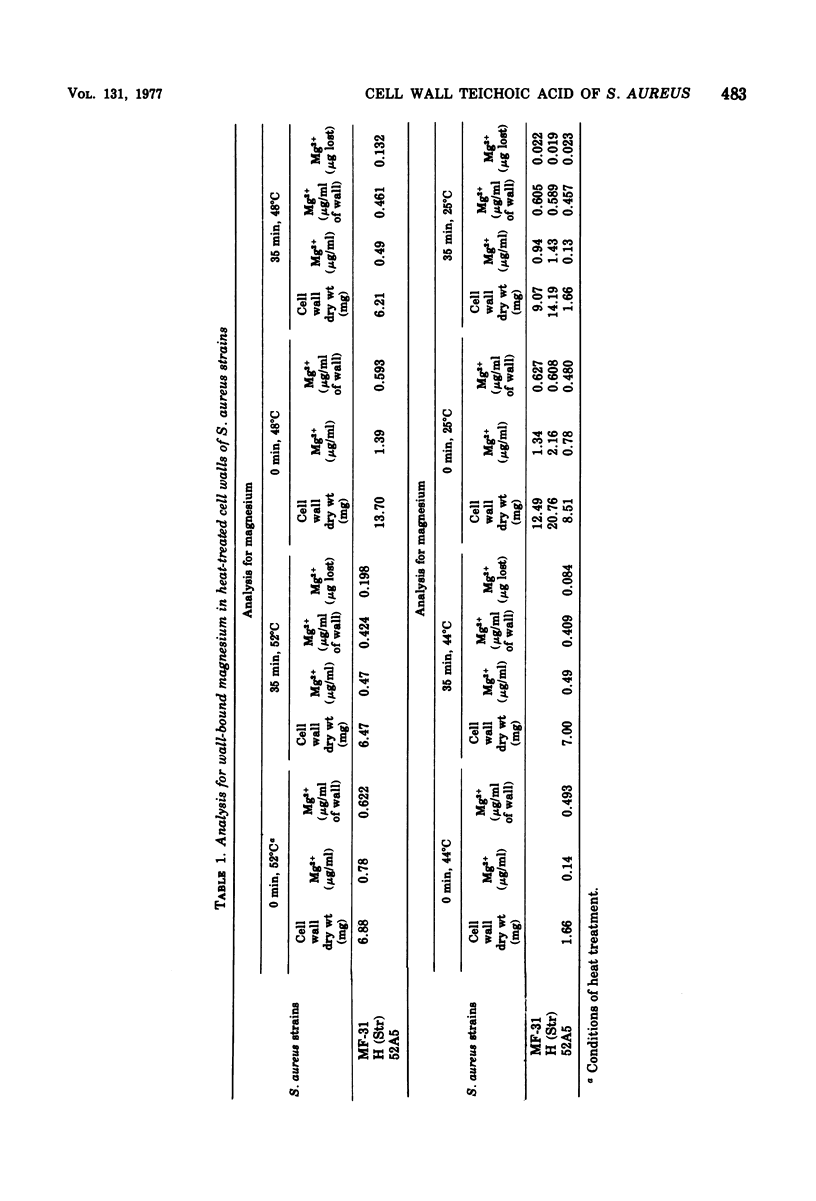
Thermally injured cells of Staphylococcus aureus lack the ability to grow on tryptic soy agar containing 7.5% NaCl. This injury phenomenon was examined in three strains of S. aureus: MF-31; H (Str); and, isolated from H (Str), 52A5, a mutant which lacks teichoic acid in the cell wall. Temperatures for sublethal heat treatment were selected to produce maximum injury with minimum death for each strain. Examination of isolated cell walls showed that magnesium was lost from the wall during heating, and that the degree of cell injury was accentuated when magnesium ions were either removed from or made unavailable to the cell. S. aureus 52A5 was more heat sensitive than its parent strain. Cells containing higher levels of wall teichoic acid generally showed less injury than normal cells. Cells with the weaker cation-binding polymer, teichuronic acid, in the cell wall generally showed greater injury. These data suggest that cell wall teichoic acid of S. aureus aids in the survival of the cell by the maintenance of an accessible surface pool of magnesium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHIBALD A. R., ARMSTRONG J. J., BADDILEY J., HAY J. B. Teichoic acids and the structure of bacterial walls. Nature. 1961 Aug 5;191:570–572. doi: 10.1038/191570a0. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Mechanism of Thermal Injury in Staphylococcus aureus: I. Relationship Between Viability and Leakage. Appl Microbiol. 1967 Nov;15(6):1266–1269. doi: 10.1128/am.15.6.1266-1269.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- Cutinelli C., Galdiero F. Ion-binding properties of the cell wall of Staphylococcus aureus. J Bacteriol. 1967 Jun;93(6):2022–2023. doi: 10.1128/jb.93.6.2022-2023.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Tempest D. W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem J. 1969 Jan;111(1):1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C. The wall content and composition of Bacillus substilis var. niger grown in a chemostat. Biochem J. 1970 Jul;118(3):367–373. doi: 10.1042/bj1180367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Young F. E., Chatterjee A. N. Characterization of a stable L-form of Bacillus subtilis 168. J Bacteriol. 1973 Jan;113(1):486–499. doi: 10.1128/jb.113.1.486-499.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C. M., Pattee P. A. Temperature-Sensitive Osmotically Fragile Mutants of Staphylococcus aureus. J Bacteriol. 1970 Dec;104(3):1401–1403. doi: 10.1128/jb.104.3.1401-1403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hurst A., Collins-Thompson D. L., Kruse H. Effect of glucose and pH on growth and enterotoxin B synthesis by Staphylococcus aureus, strain S6, after heat injury in sodium or potassium phosphate buffer. Can J Microbiol. 1973 Jul;19(7):823–829. doi: 10.1139/m73-132. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Beare-Rogers J. L., Collins-Thompson D. L. Pysiological studies on the recovery of salt tolerance by Staphylococcus aureus after sublethal heating. J Bacteriol. 1973 Nov;116(2):901–907. doi: 10.1128/jb.116.2.901-907.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Collins-Thompson D. L., Shah B. G. Relationship between loss of magnesium and loss of salt tolerance after sublethal heating of Staphylococcus aureus. Can J Microbiol. 1974 Aug;20(8):1153–1158. doi: 10.1139/m74-178. [DOI] [PubMed] [Google Scholar]
- Hurst A., Hughes A., Duckworth M., Baddiley J. Loss of D-alanine during sublethal heating of Staphylococcus aureus S6 and magnesium binding during repair. J Gen Microbiol. 1975 Aug;89(2):277–284. doi: 10.1099/00221287-89-2-277. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemasa Y., Yoshioka T., Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureus cultured in medium containing NaCl. Biochim Biophys Acta. 1972 Nov 30;280(3):444–450. [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. The interaction of magnesium ions with teichoic acid. Biochem J. 1975 Sep;149(3):519–524. doi: 10.1042/bj1490519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Permeability of the envelopes of Staphylococcus aureus to some salts, amino acids, and non-electrolytes. J Gen Microbiol. 1959 Apr;20(2):434–441. doi: 10.1099/00221287-20-2-434. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Mayzel K., Carstensen E. L. Cation exchange in cell walls of gram-positive bacteria. Can J Microbiol. 1976 Jul;22(7):975–982. doi: 10.1139/m76-142. [DOI] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W. The influence of growth-limiting substrate and medium NaCl concentration on the synthesis of magnesium-binding sites in the walls of Bacillus subtilis var. niger. J Gen Microbiol. 1970 Nov;63(3):325–331. doi: 10.1099/00221287-63-3-325. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Chatterjee A. N., Young F. E., Marquis R. E. The physiology of teichoic acid deficient staphylococci. Can J Microbiol. 1973 Nov;19(11):1393–1399. doi: 10.1139/m73-225. [DOI] [PubMed] [Google Scholar]
- STILES M. E., WITTER L. D. THERMAL INACTIVATION, HEAT INJURY, AND RECOVERY OF STAPHYLOCOCCUS AUREUS. J Dairy Sci. 1965 Jun;48:677–681. doi: 10.3168/jds.s0022-0302(65)88321-7. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., SHON M. EFFECTS OF THERMAL STRESS ON VIABILITY AND RIBONUCLEIC ACID OF AEROBACTER AEROGENES IN AQUEOUS SUSPENSION. J Gen Microbiol. 1964 Jan;34:99–114. doi: 10.1099/00221287-34-1-99. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Ellwood D. C. Influence of growth condition on the concentration of potassium in Bacillus subtilis var. niger and its possible relationship to cellular ribonucleic acid, teichoic acid and teichuronic acid. Biochem J. 1968 Jan;106(1):237–243. doi: 10.1042/bj1060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Meers J. L. Magnesium-limited growth of Bacillus subtilis, in pure and mixed cultures, in a chemostat. J Gen Microbiol. 1967 Oct;49(1):139–147. doi: 10.1099/00221287-49-1-139. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Strange R. E. Variation in content and distribution of magnesium, and its influence on survival, in Aerobacter aerogenes grown in a chemostat. J Gen Microbiol. 1966 Aug;44(2):273–279. doi: 10.1099/00221287-44-2-273. [DOI] [PubMed] [Google Scholar]


