Abstract
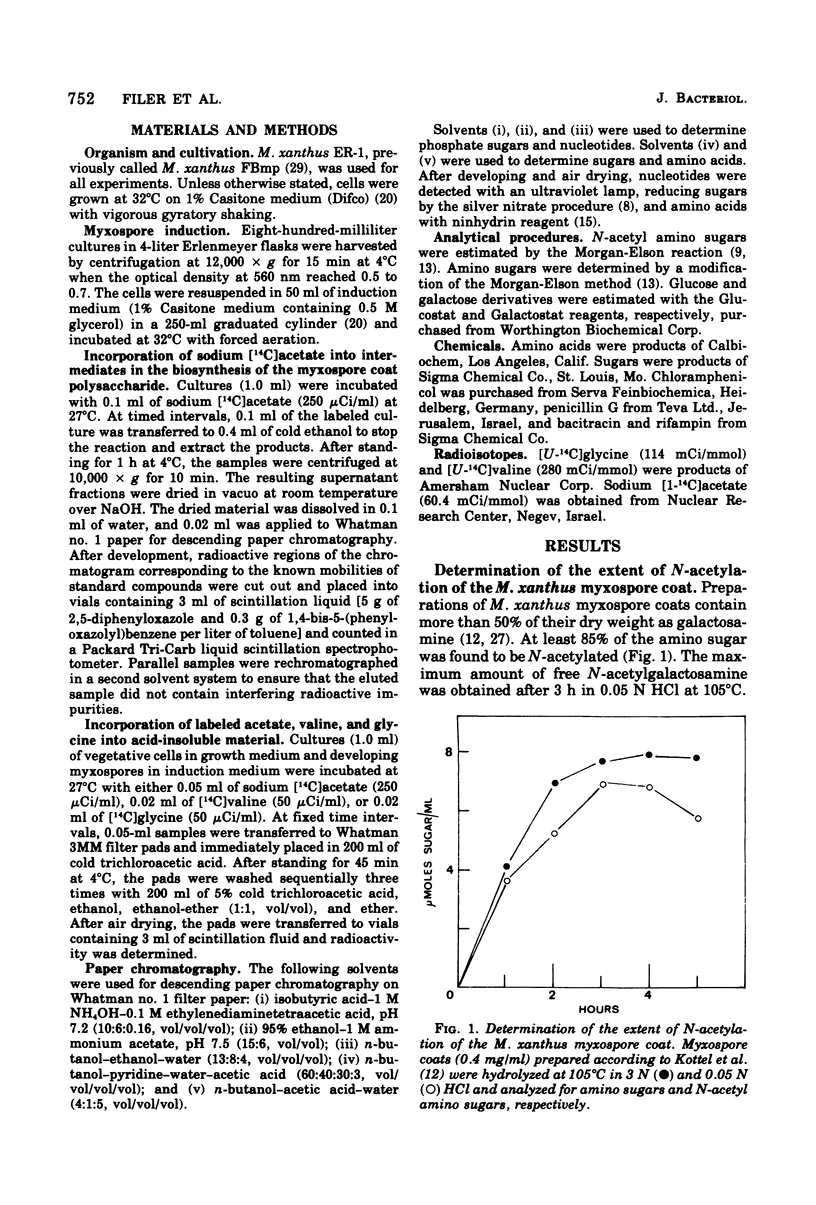
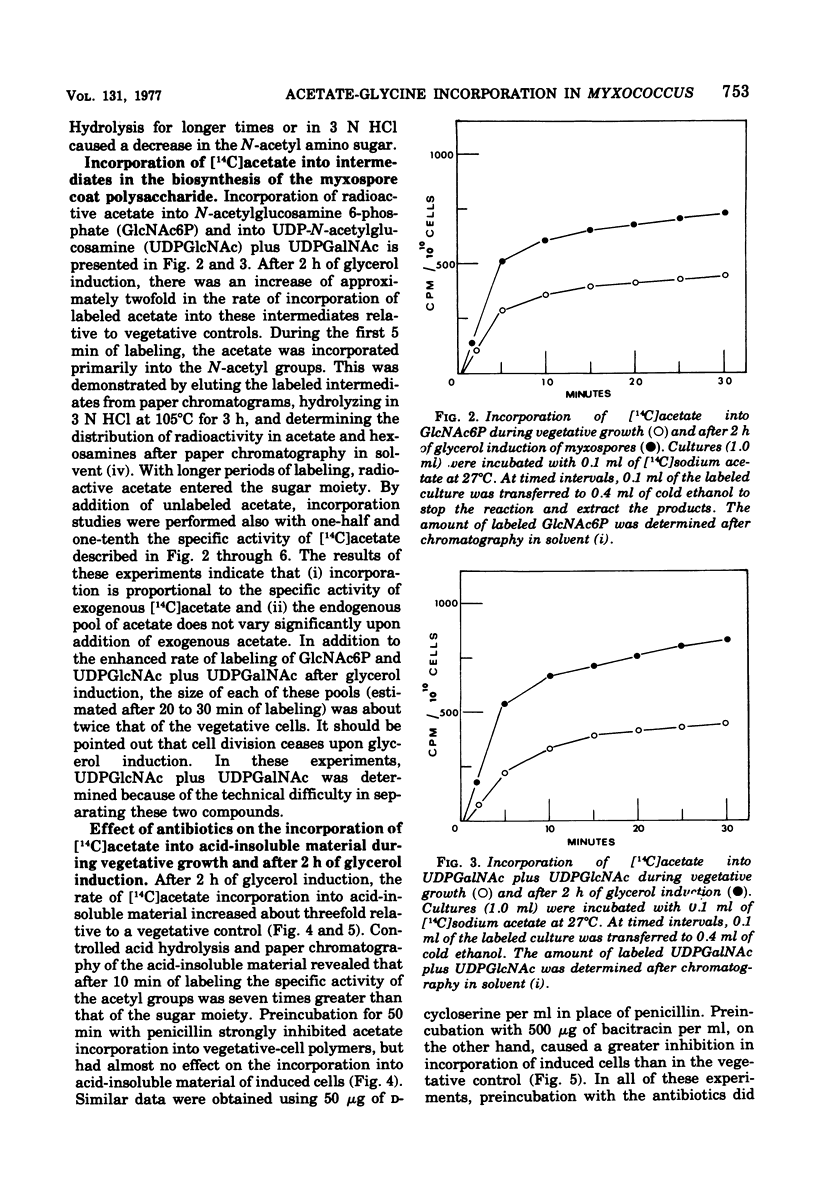
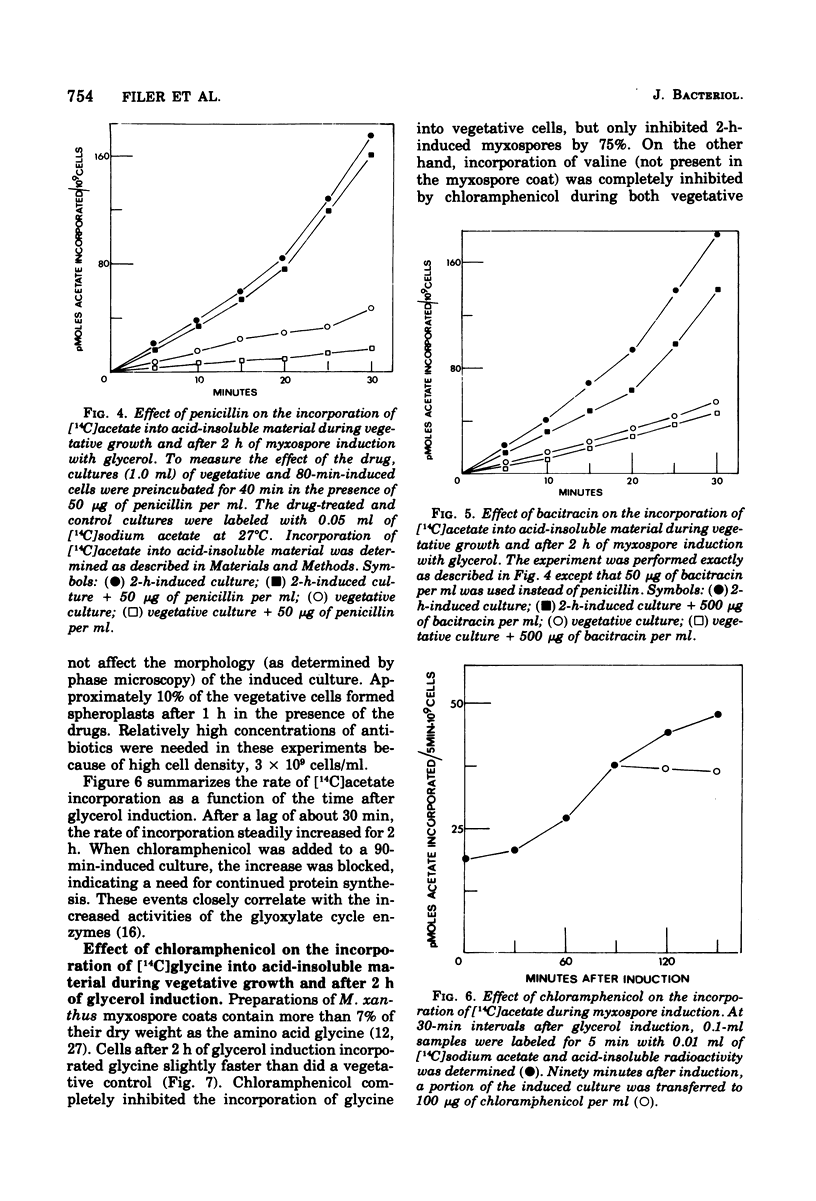
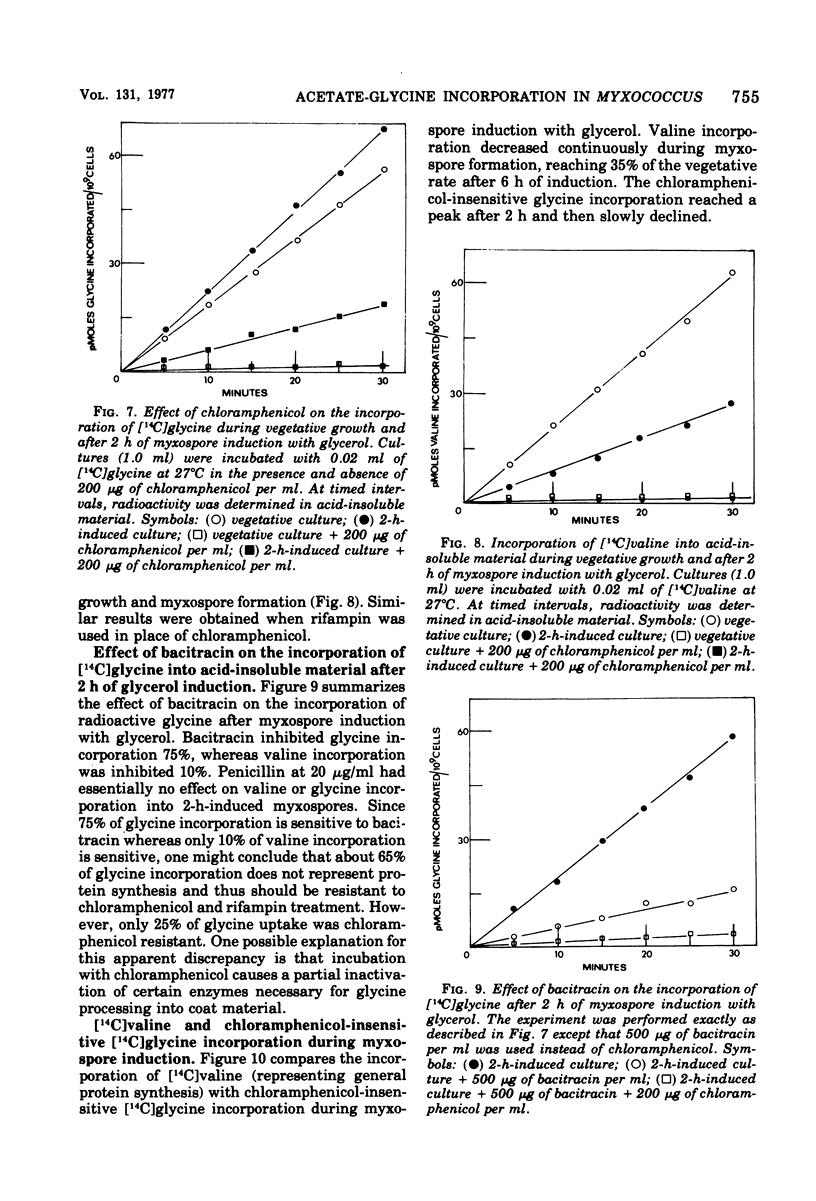
Myxospore coat synthesis in Myxococcus xanthus was studied by incorporation of [14C]acetate into intermediates in the biosynthesis of coat polysaccharide and into acid-insoluble material during vegetative growth and after glycerol induction of myxospores. During short labeling periods at 27°C, the radioactivity was shown to be located primarily in N-acetyl groups rather than sugar moieties. Two hours after glycerol induction, the pools of N-acetylglucosamine 6-phosphate and uridine 5′-diphosphate-N-acetylgalactosamine (UDPGalNAc) plus uridine 5′-diphosphate-N-glucosamine increased about twofold and were labeled at twice the rate measured for vegetative cells. The increased rate of synthesis of UDPGalNAc and its precursors could be correlated with increased enzyme activities measured in vitro. Controlled acid hydrolysis revealed that the galactosamine portion of the myxospore coat was N-acetylated. After glycerol induction, the incorporation of acetate into acid-insoluble material increased threefold. This enhanced incorporation was sensitive to neither penicillin nor d-cycloserine. In contrast, bacitracin inhibited the incorporation of [14C]acetate into acid-insoluble material more effectively 2 h after myxospore induction than during vegetative growth. Chloramphenicol added to cells 90 min after induction blocked further increase in the rate of [14C]acetate incorporation. Since the myxospore coat contains glycine, polymer synthesis was also measured by chloramphenicol-insensitive [14C]glycine incorporation into acid-insoluble material. Although protein synthesis decreased after glycerol induction, glycine incorporation increased. Two hours after induction, glycine incorporation was only 75% inhibited by chloramphenicol and rifampin. The chloramphenicol-insensitive rate of incorporation of [14C]glycine increased during the first hour after myxospore induction and reached a peak rate after 2 to 3 h. The chloramphenicol-resistant incorporation of [14C]glycine was resistant to penicillin but sensitive to bacitracin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Sussman M. The appearance and disappearance of uridine diphosphate glucose pyrophosphorylase activity during differntiation of the cellular slime mold Dictyostelium discoideum. J Biol Chem. 1967 Apr 25;242(8):1696–1700. [PubMed] [Google Scholar]
- Bacon K., Eiserling F. A. A unique structure in microcysts of Myxococcus xanthus. J Ultrastruct Res. 1967 Dec;21(5):378–382. doi: 10.1016/s0022-5320(67)80147-3. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Filer D., Kindler S. H., Rosenberg E. Myxospore coat synthesis in Myxococcus xanthus: enzymes associated with uridine 5'-diphosphate-N-acetylgalactosamine formation during myxospore development. J Bacteriol. 1977 Sep;131(3):745–750. doi: 10.1128/jb.131.3.745-750.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J., Sussman M. Accumulation of uridine diphosphoglucose pyrophosphorylase in Dictyostelium discoideum via preferential synthesis. J Mol Biol. 1973 Dec 5;81(2):173–185. doi: 10.1016/0022-2836(73)90187-3. [DOI] [PubMed] [Google Scholar]
- Franke J., Sussman M. Synthesis of uridine diphosphate glucose pyrophosphorylase during the development of Dictyostelium discoideum. J Biol Chem. 1971 Nov;246(21):6381–6388. [PubMed] [Google Scholar]
- Gal A. E. Separation and identification of monosaccharides from biological materials by thin-layer chromatography. Anal Biochem. 1968 Sep;24(3):452–461. doi: 10.1016/0003-2697(68)90152-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. Y., White D. Myxospore formation in Myxococcus xanthus: chemical changes in the cell wall during cellular morphogenesis. J Bacteriol. 1972 Nov;112(2):849–855. doi: 10.1128/jb.112.2.849-855.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick K. A., Wright B. E. Regulation of enzyme activity during differentiation in Dictyostelium discoideum. Annu Rev Microbiol. 1974;28(0):139–166. doi: 10.1146/annurev.mi.28.100174.001035. [DOI] [PubMed] [Google Scholar]
- Kottel R. H., Bacon K., Clutter D., White D. Coats from Myxococcus xanthus: characterization and synthesis during myxospore differentiation. J Bacteriol. 1975 Oct;124(1):550–557. doi: 10.1128/jb.124.1.550-557.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Martin P., White D., Wong M. C. Changes in activity of glyoxylate cycle enzymes during myxospore development in Myxococcus xanthus. J Bacteriol. 1972 Sep;111(3):784–790. doi: 10.1128/jb.111.3.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., White D. Inactivation of isocitrate lyase during myxospore development in Myxococcus xanthus. J Bacteriol. 1974 Apr;118(1):96–102. doi: 10.1128/jb.118.1.96-102.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Vaks B., Zuckerberg A. Bactericidal action of an antibiotic produced by Myxococcus xanthus. Antimicrob Agents Chemother. 1973 Nov;4(5):507–513. doi: 10.1128/aac.4.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler W., Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966 Apr;91(4):1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R. Mechanism of bacitracin action: a specific lipid-peptide interaction. Ann N Y Acad Sci. 1974 May 10;235(0):387–398. doi: 10.1111/j.1749-6632.1974.tb43278.x. [DOI] [PubMed] [Google Scholar]
- Troy F. A., Frerman F. E., Heath E. C. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J Biol Chem. 1971 Jan 10;246(1):118–133. [PubMed] [Google Scholar]
- VOELZ H., DWORKIN M. Fine structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962 Nov;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaks B., Zuckerberg A., Rosenberg E. Purification and partial characterization of an antibiotic produced by Myxococcus xanthus. Can J Microbiol. 1974 Feb;20(2):155–161. doi: 10.1139/m74-025. [DOI] [PubMed] [Google Scholar]
- Watson B. F., Dworkin M. Comparative intermediary metabolism of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1968 Nov;96(5):1465–1473. doi: 10.1128/jb.96.5.1465-1473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. E., Gustafson G. L. Expansion of the kinetic model of differentiation in Dictyostelium discoideum. J Biol Chem. 1972 Dec 25;247(24):7875–7884. [PubMed] [Google Scholar]
- Wright B. E. Multiple causes and controls in differentiation. Science. 1966 Aug 19;153(3738):830–837. doi: 10.1126/science.153.3738.830. [DOI] [PubMed] [Google Scholar]


