Abstract
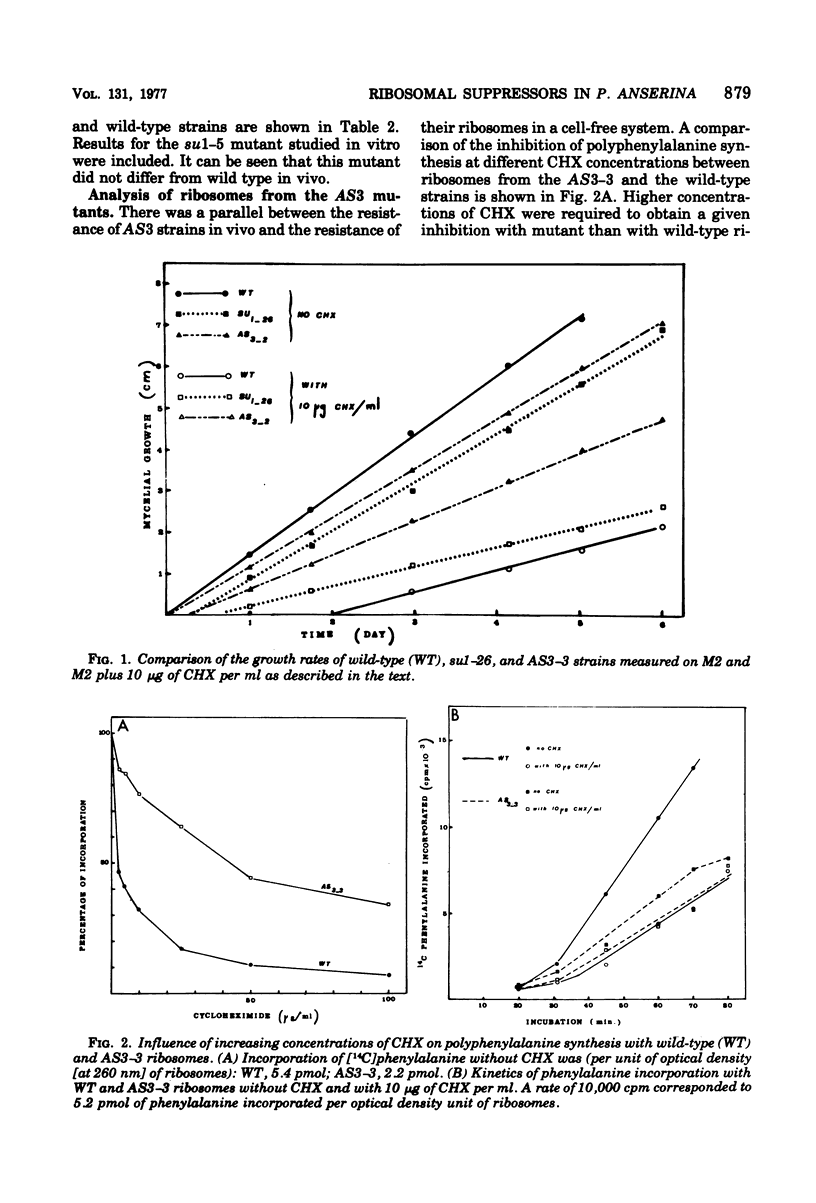
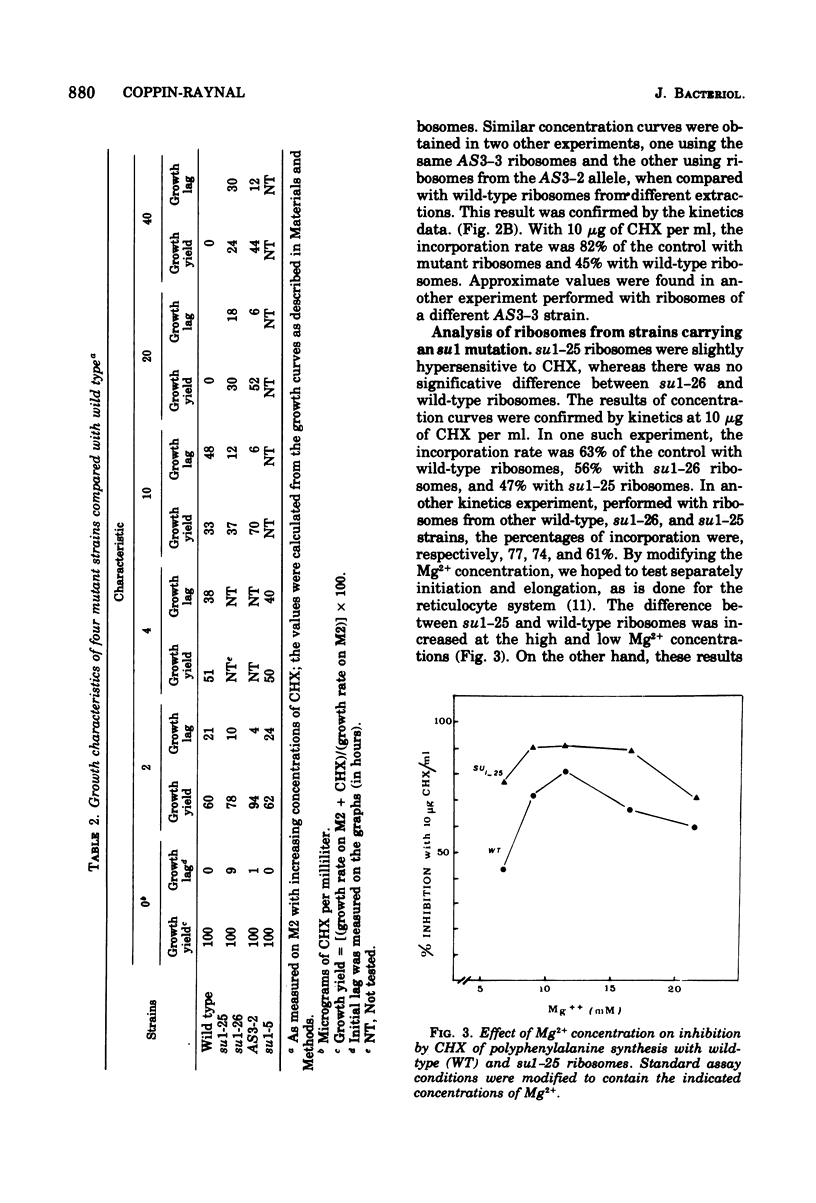
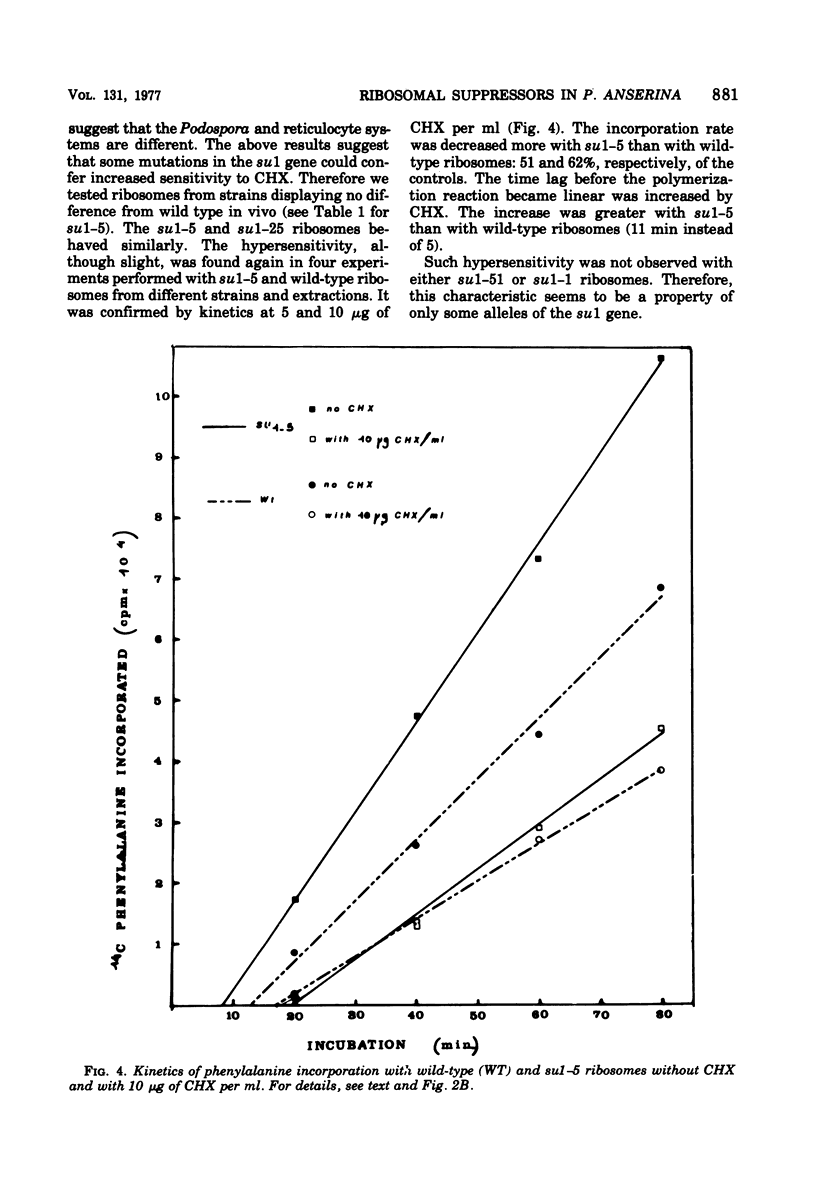
Informational suppressors and antisuppressors have been previously isolated in Podospora anserina, and a range of exclusively genetic arguments have led to the assumption that they correspond to ribosomal mutations. An in vivo and in vitro comparison of the effect of the ribosomal inhibitor cycloheximide on wildtype and mutant strains described in this paper confirms the ribosomal hypothesis for at least some mutants. Indeed, the four mutants in the AS3 gene were cycloheximide resistant, and their ribosomes were found to be resistant when analyzed by polyuridyl-directed polyphenylalanine systhesis. On the other hand, ribosomes from two su 1 mutants were hypersensitive to the drug.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Saltzman L. Functional interdependence of 50S and 30S ribosomal subunits. Mol Gen Genet. 1974;135(1):11–18. doi: 10.1007/BF00433896. [DOI] [PubMed] [Google Scholar]
- Bayliss F. T., Ingrahm J. L. Mutation in Saccharomyces cerevisiae conferring streptomycin and cold sensitivity by affecting ribosome formation and function. J Bacteriol. 1974 May;118(2):319–328. doi: 10.1128/jb.118.2.319-328.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Banthorpe D. V., Wilkie D. Modified ribosomes conferring resistance to cycloheximide in mutants of Saccharomyces cerevisiae. J Mol Biol. 1967 Jun 14;26(2):347–350. doi: 10.1016/0022-2836(67)90302-6. [DOI] [PubMed] [Google Scholar]
- Elseviers D., Gorini L. Direct selection of mutants restricting efficiency of suppression and misreading levels in E. coli B. Mol Gen Genet. 1975;137(4):277–287. doi: 10.1007/BF00703254. [DOI] [PubMed] [Google Scholar]
- Garvin R. T., Gorini L. A new gene for ribosomal restriction in Escherichia coli. Mol Gen Genet. 1975;137(1):73–78. doi: 10.1007/BF00332540. [DOI] [PubMed] [Google Scholar]
- Grant P., Sánchez L., Jiménez A. Cryptopleurine resistance: genetic locus for a 40S ribosomal component in Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1308–1314. doi: 10.1128/jb.120.3.1308-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. A., Coddington A. Genetic studies on cycloheximide-resistant strains of Schizosaccharomyces pombe. Heredity (Edinb) 1976 Oct;37(2):179–191. doi: 10.1038/hdy.1976.81. [DOI] [PubMed] [Google Scholar]
- Neuhäuser A., Klingmüller W., Kaudewitz F. Selektion Actidion-resistenter Mutanten bei Neurospora crassa sowie ihre genetische und biochemische Analyse. Mol Gen Genet. 1970;106(2):180–194. doi: 10.1007/BF00323837. [DOI] [PubMed] [Google Scholar]
- Obrig T. G., Culp W. J., McKeehan W. L., Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem. 1971 Jan 10;246(1):174–181. [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Picard-Bennoun M. Genetic evidence of ribosomal antisuppressors in Podospora anserina. Mol Gen Genet. 1976 Sep 23;147(3):299–306. doi: 10.1007/BF00582881. [DOI] [PubMed] [Google Scholar]
- Pongratz M., Klingmüller W. Role of ribosomes in cycloheximide resistance of Neurospora mutants. Mol Gen Genet. 1973 Aug 28;124(4):359–363. doi: 10.1007/BF00267664. [DOI] [PubMed] [Google Scholar]
- Rao S. S., Grollman A. P. Cycloheximide resistance in yeast: a property of the 60s ribosomal subunit. Biochem Biophys Res Commun. 1967 Dec 15;29(5):696–704. doi: 10.1016/0006-291x(67)90273-2. [DOI] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Schindler D., Grant P., Davies J. Trichodermin resistance--mutation affecting eukaryotic ribosomes. Nature. 1974 Apr 5;248(448):535–536. doi: 10.1038/248535a0. [DOI] [PubMed] [Google Scholar]
- Schlitt S. C., Russell P. J. Neurospora crassa cytoplasmic ribosomes: isolation and characterization of a cold-sensitive mutant defective in ribosome biosynthesis. J Bacteriol. 1974 Nov;120(2):666–671. doi: 10.1128/jb.120.2.666-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M. R., Sisler H. D. Site of action of cycloheximide in cells of Saccharomyces pastorianus. 3. Further studies on the mechanism of action and the mechanism of resistance in saccharomyces species. Biochim Biophys Acta. 1965 Aug 10;103(4):558–567. [PubMed] [Google Scholar]
- Skogerson L., McLaughlin C., Wakatama E. Modification of ribosomes in cryptopleurine-resistant mutants of yeast. J Bacteriol. 1973 Nov;116(2):818–822. doi: 10.1128/jb.116.2.818-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P., Minet M., Hofer F., Leupold U. Genetic analysis of antisuppressor mutants in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Dec 31;142(4):251–261. doi: 10.1007/BF00271250. [DOI] [PubMed] [Google Scholar]
- Waldron C., Roberts C. F. Cold-sensitive mutants in Aspergillus nidulans. II. Mutations affecting ribosome production. Mol Gen Genet. 1974;134(2):115–132. doi: 10.1007/BF00268414. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G., Apirion D., Rosen L., Tanaka K., Tamaki M., Takata R., Dekio S., Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973 Dec 20;127(2):175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Garvin R. T., Gorini L. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2263–2267. doi: 10.1073/pnas.68.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]


