Abstract
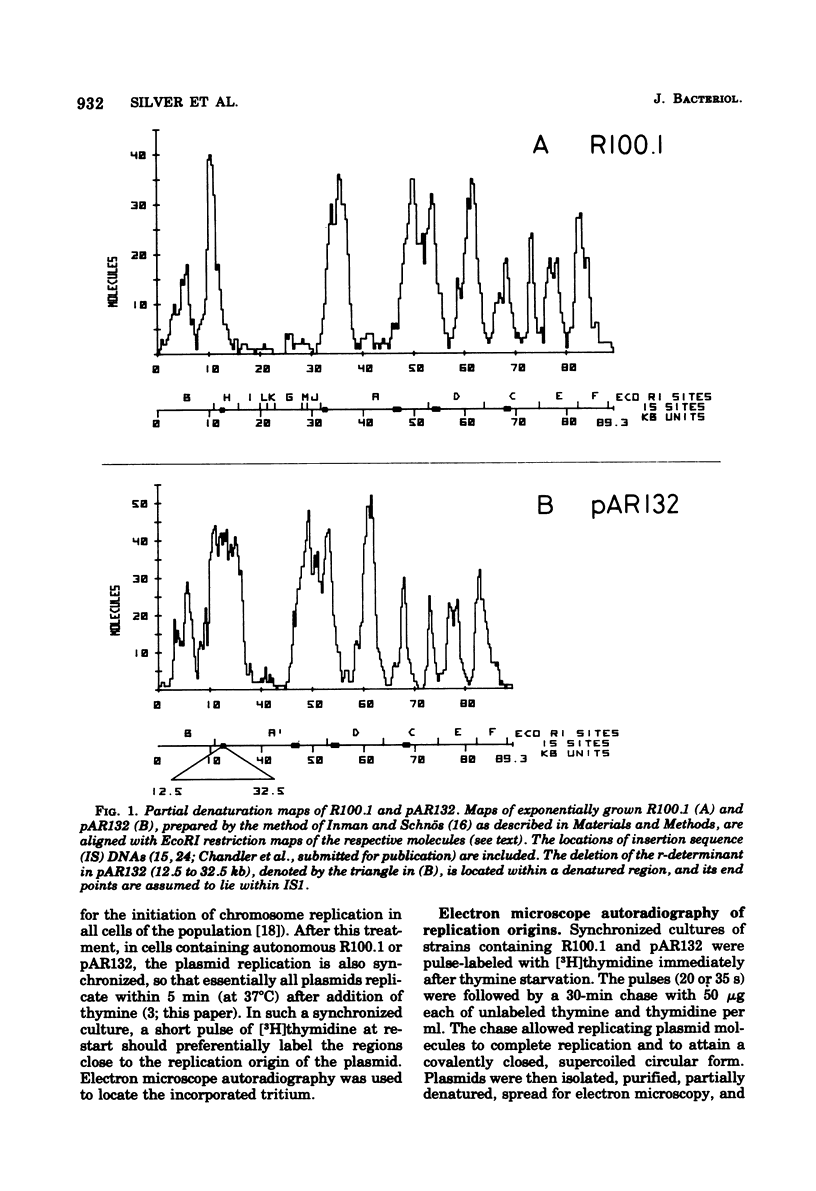
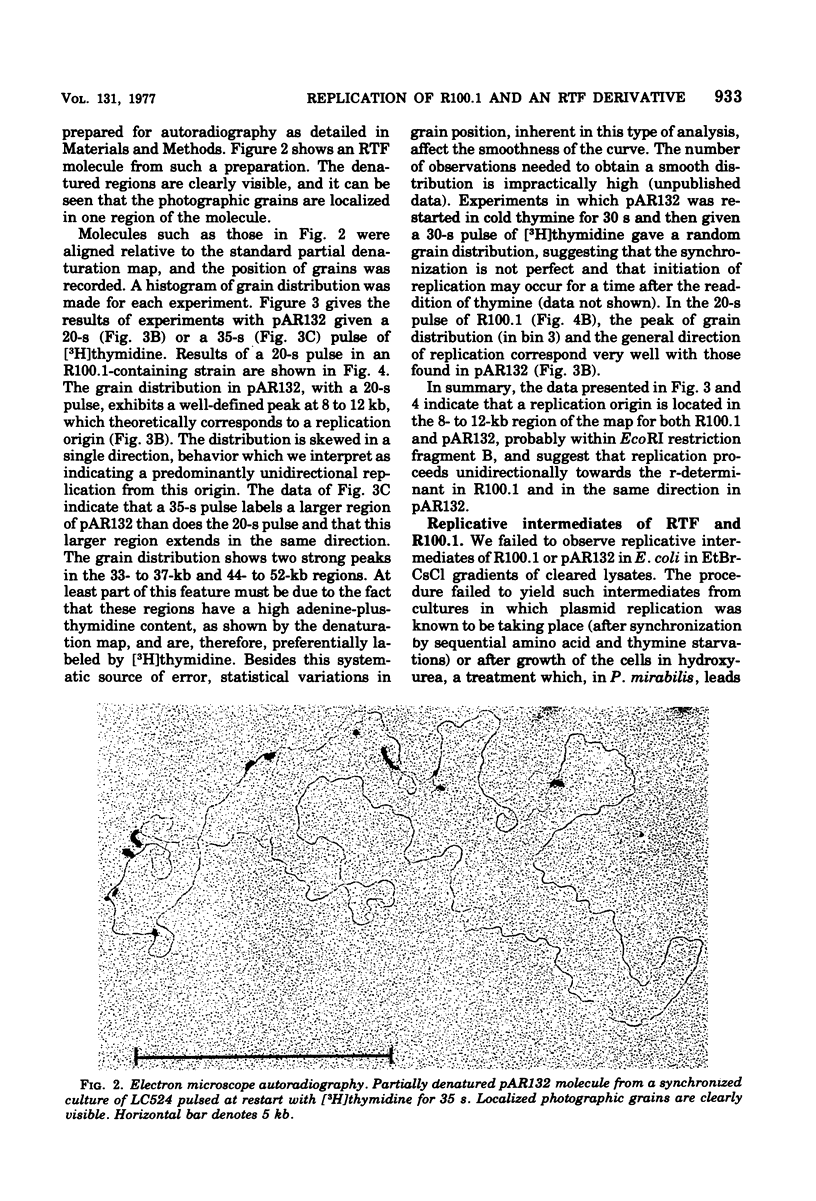
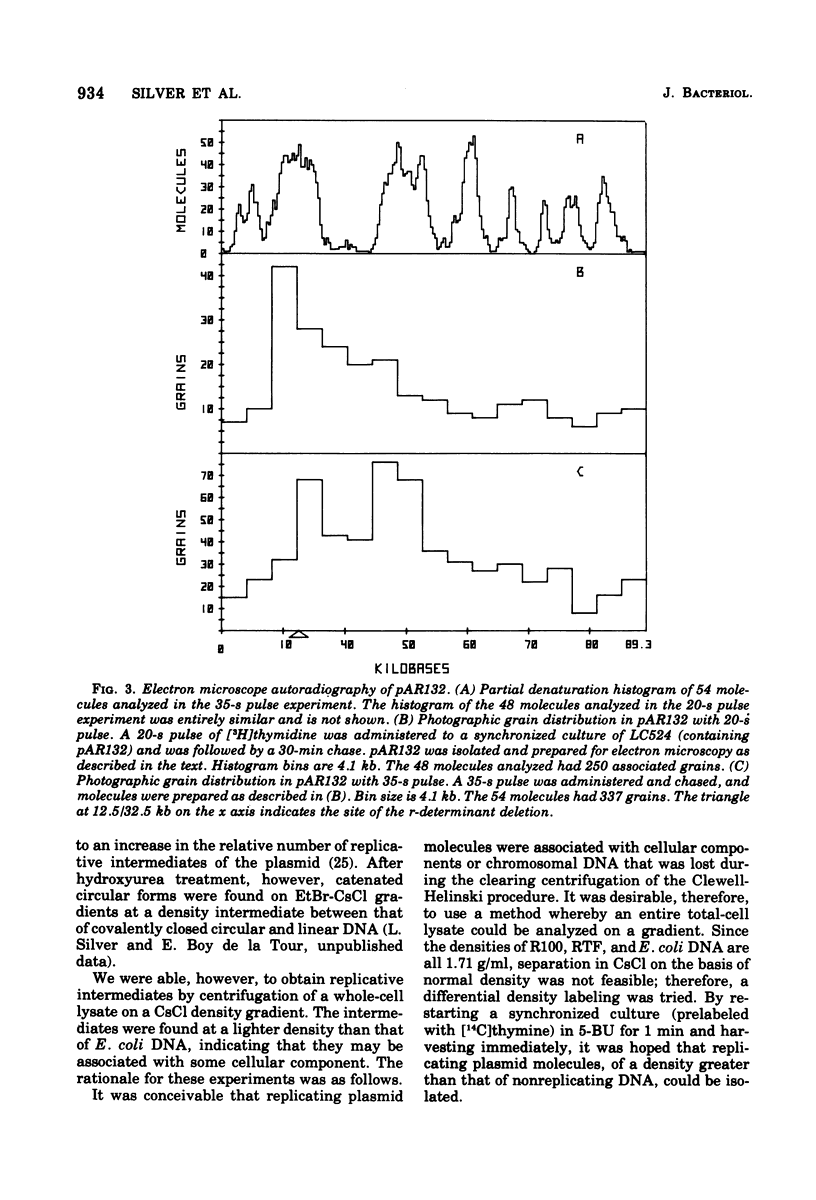
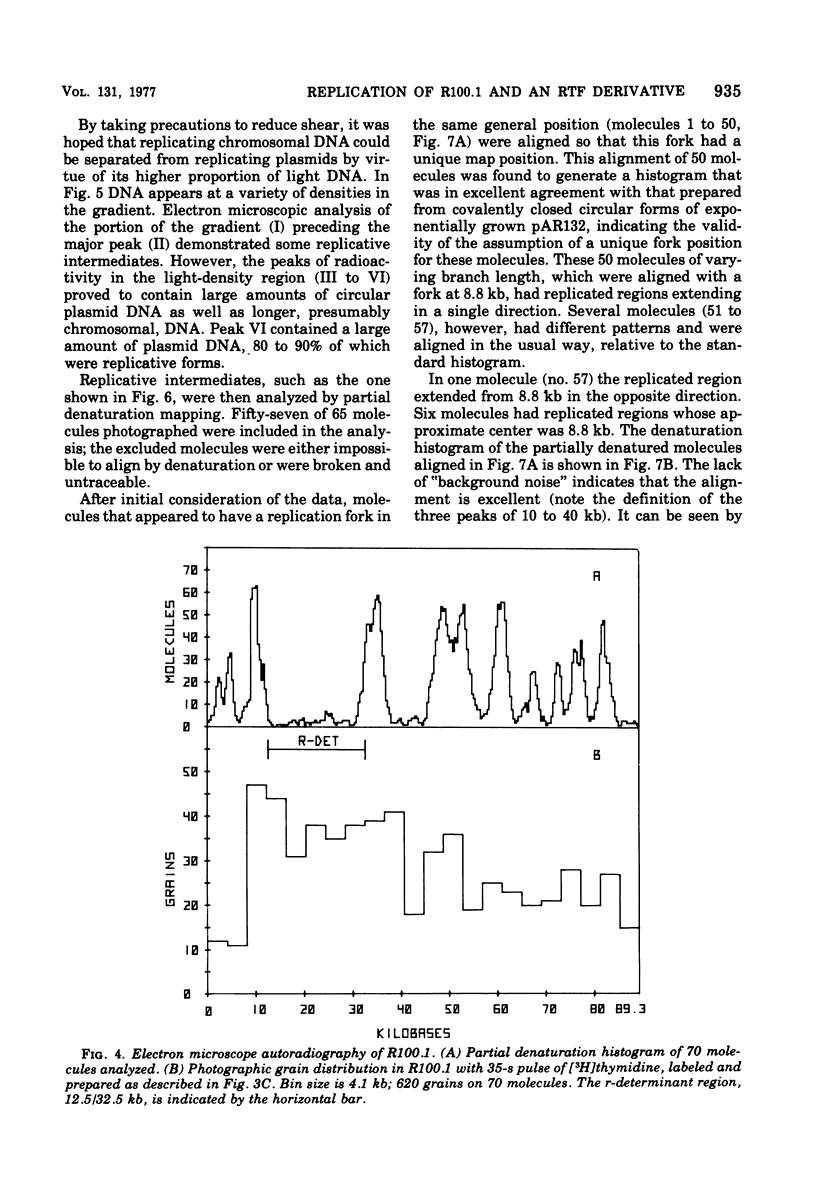
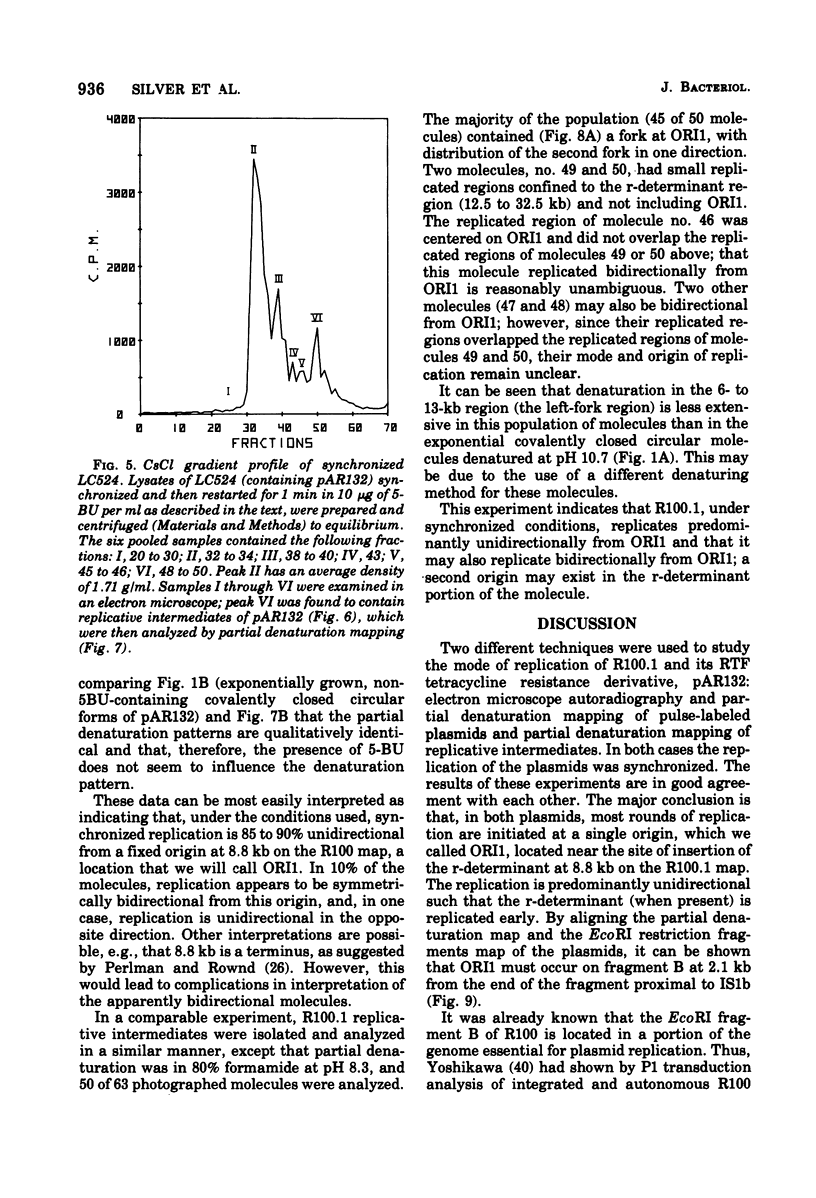
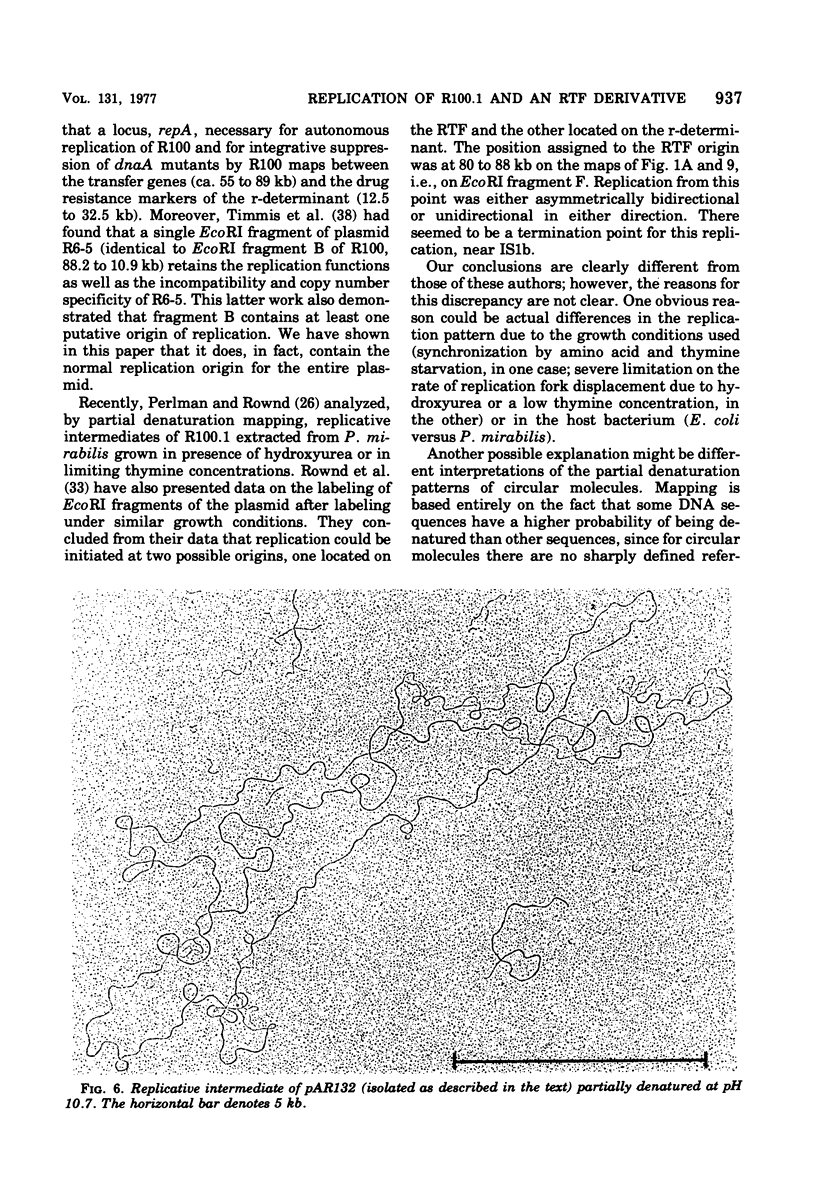
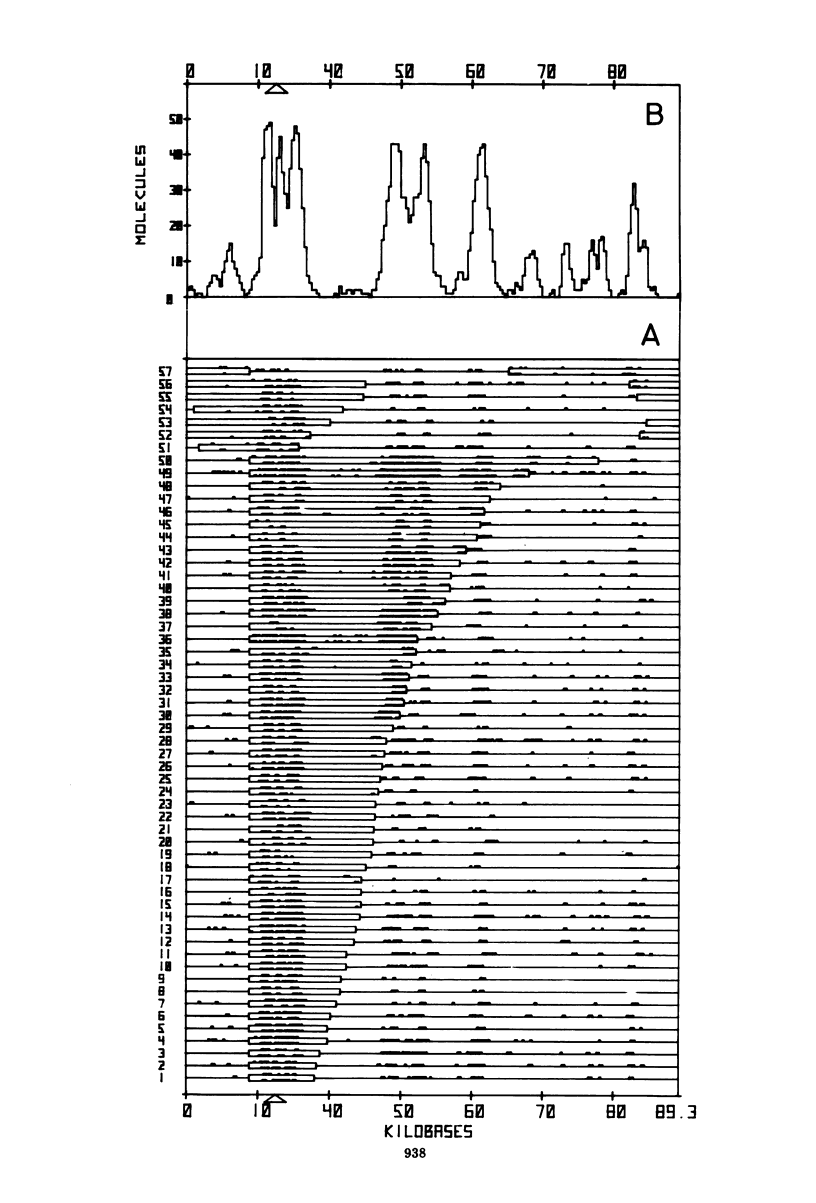
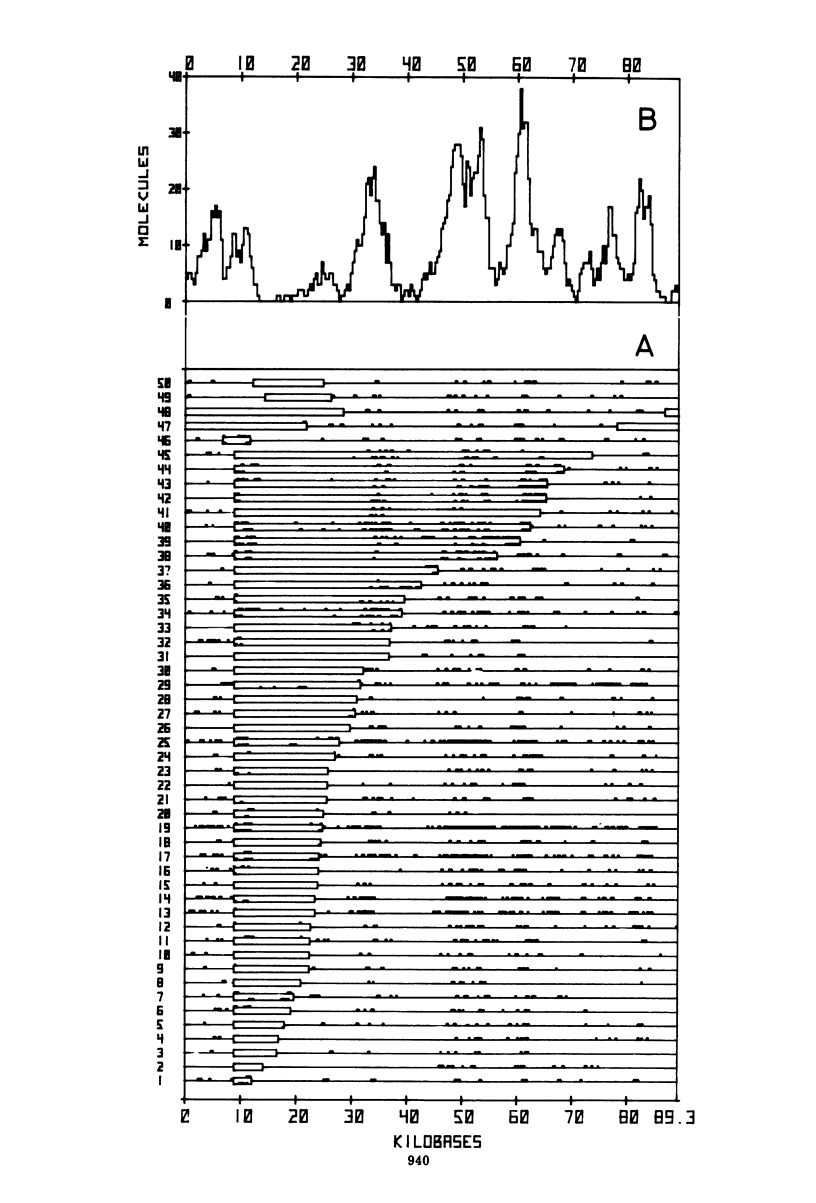
The origin and direction of replication of the resistance plasmid R100.1 and its resistance transfer factor derivative, pAR132, were studied by electron microscopy autoradiography of partially denatured molecules and partial denaturation mapping of replicative intermediates. Results of these studies indicate the existence of an origin of replication at 8.8 kilobases on the R100 map. Replication from this origin in cultures synchronized for initiation of replication is predominantly unidirectional in a single direction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Chandler M., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R.100.1: Change of chromosome replication origin in synchronized cultures. J Bacteriol. 1976 Jun;126(3):1215–1223. doi: 10.1128/jb.126.3.1215-1223.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Silver L., Frey J., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R100.1: generation of small plasmids after integration. J Bacteriol. 1977 Apr;130(1):303–311. doi: 10.1128/jb.130.1.303-311.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. 3. Isolation of the discrete transfer unit of the R-factor R1. Proc Natl Acad Sci U S A. 1970 Oct;67(2):510–516. doi: 10.1073/pnas.67.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Mode of replication of the conjugative R-plasmid RSF1040 in Escherichia coli. J Bacteriol. 1976 Apr;126(1):454–466. doi: 10.1128/jb.126.1.454-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- Louarn J., Funderburgh M., Bird R. E. More precise mapping of the replication origin in Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):1–5. doi: 10.1128/jb.120.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M. A., Sparks R. B., Helinski D. R. Bidirectional replication of plasmid R6K DNA in Escherichia coli; correspondence between origin of replication and position of single-strand break in relaxed complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2905–2909. doi: 10.1073/pnas.72.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- Nishimura A., Nishimura Y., Caro L. Isolation of Hfr strains from R+ and ColV2+ strains of Escherichia coli and derivation of an R'lac factor by transduction. J Bacteriol. 1973 Dec;116(3):1107–1112. doi: 10.1128/jb.116.3.1107-1112.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Isolation of inverted repeat sequences, including IS1, IS2, and IS3, in Escherichia coli plasmids. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2316–2320. doi: 10.1073/pnas.73.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Accumulation of replicating bacterial plasmid DNA during thymine limitation or hydroxyurea treatment. Mol Gen Genet. 1975 Jul 10;138(4):281–291. doi: 10.1007/BF00264797. [DOI] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Two origins of replication in composite R plasmid DNA. Nature. 1976 Jan 29;259(5541):281–284. doi: 10.1038/259281a0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Twose T. M., Holland M. J., Rownd R. H. Denaturation mapping of R factor deoxyribonucleic acid. J Bacteriol. 1975 Sep;123(3):1035–1042. doi: 10.1128/jb.123.3.1035-1042.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punch J. D., Kopecko D. J. Positive and negative control of R-factor replication in Proteus mirabilis. J Bacteriol. 1972 Jan;109(1):336–349. doi: 10.1128/jb.109.1.336-349.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Tanak N., Cramer J. H., Rownd R. H. EcoRI restriction endonuclease map of the composite R plasmid NR1. J Bacteriol. 1976 Jul;127(1):619–636. doi: 10.1128/jb.127.1.619-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M. Identification and mapping of the replication genes of an R factor, R100-1, integrated into the chromosome of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):1123–1131. doi: 10.1128/jb.118.3.1123-1131.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




