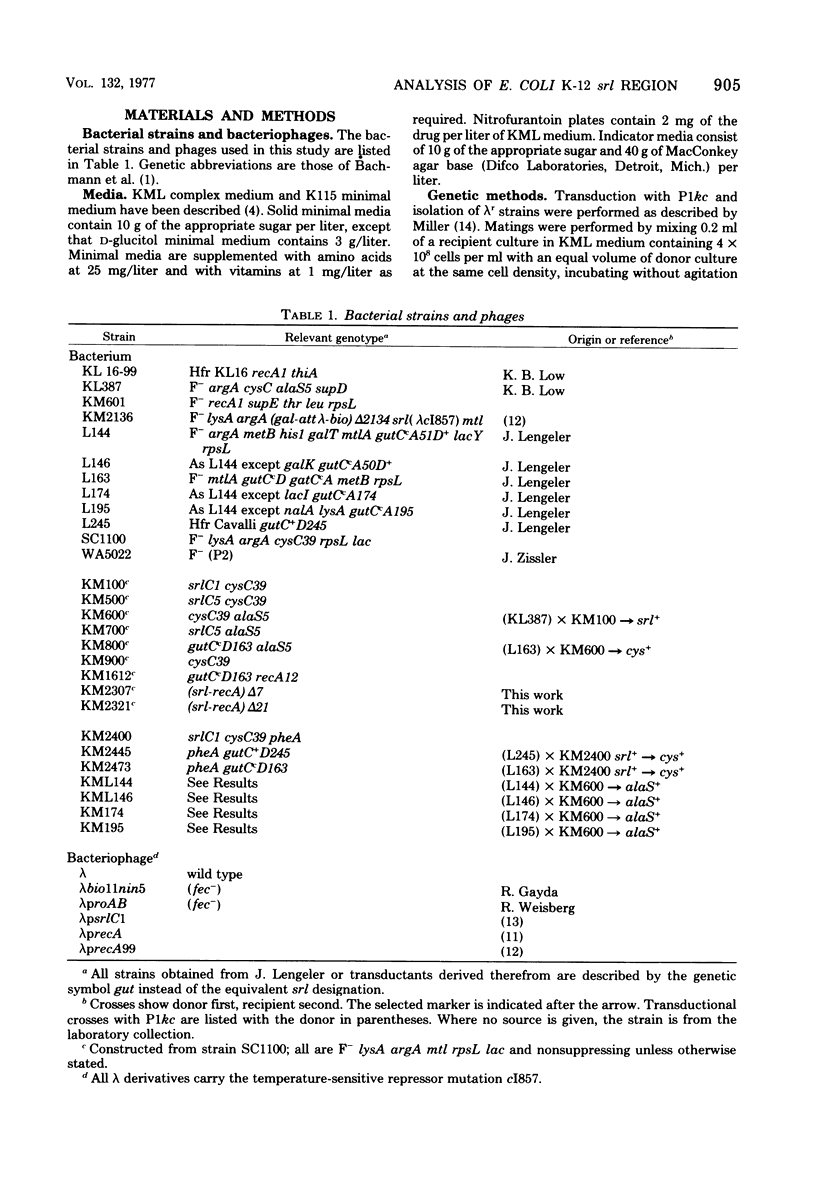
Abstract
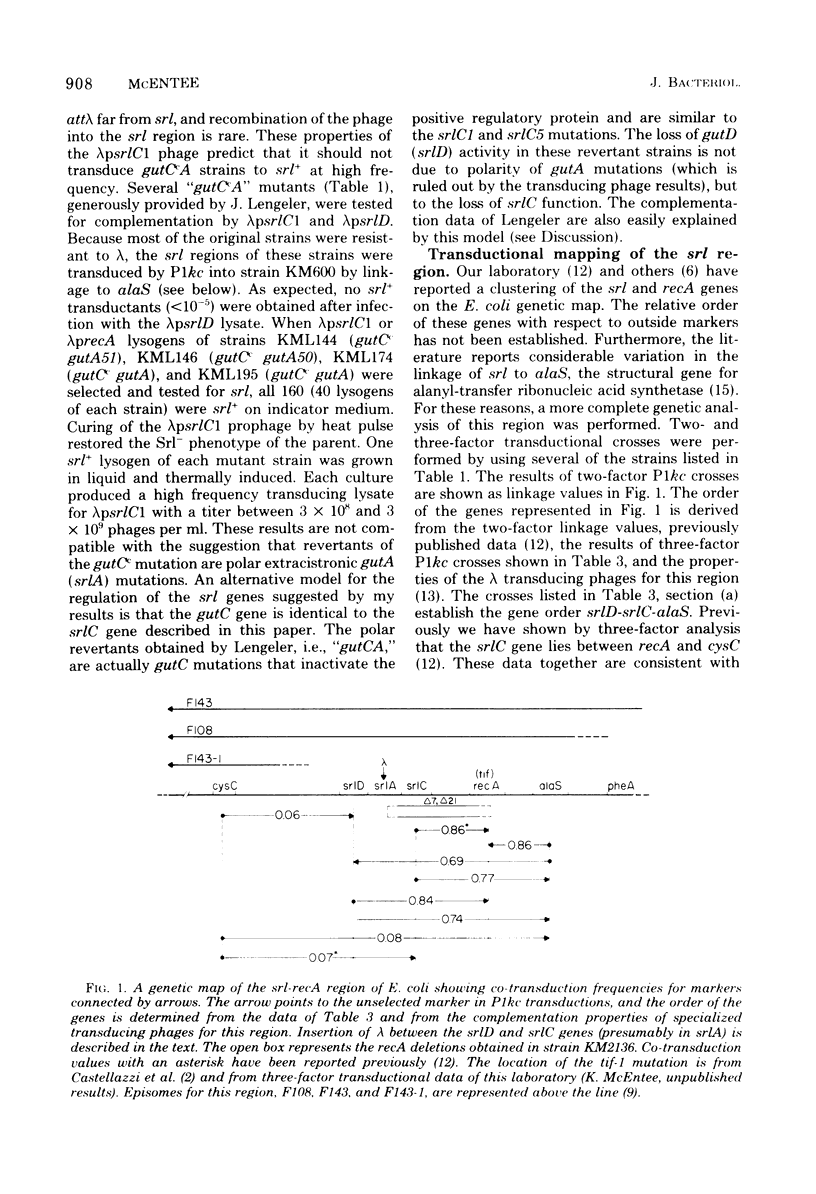
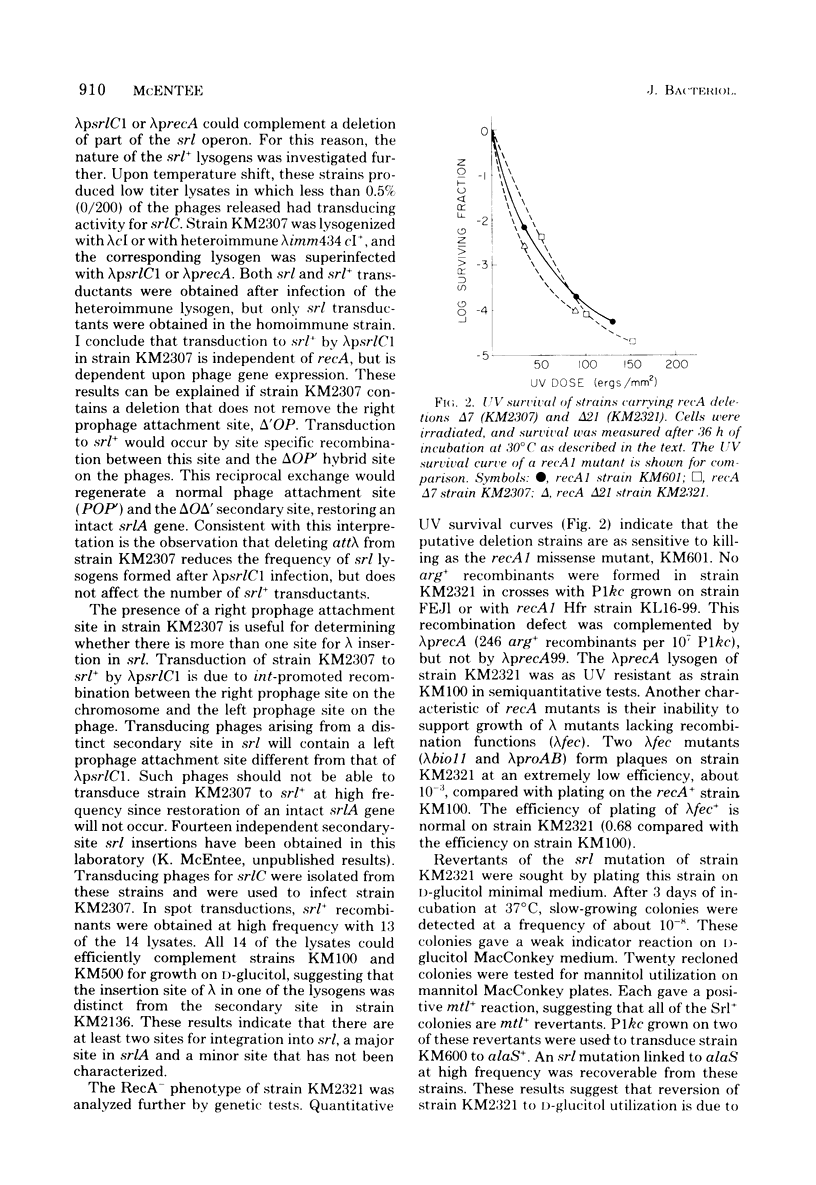
Specialized transducing lambda derivatives, deletion mapping, and Plkc transductional crosses have been used to analyze the genetic organization and regulation of the srl genes. Transducing phages obtained from a secondary site lambda insertion in srlA are of two types: lambdapsrlC1 and lambdaprecA are substituted in the b2 region of the lambda chromosome (galtype) and carry the srlC gene but not srlD; lambdapsrlD is substituted in the early region of the phage deoxyribonucleic acid (biotype) and carries the srlD gene but not srlC. The lambdapsrlC1 phage, which lysogenizes at attlambda, complements srlC mutants in trans, indicating that this gene codes for a diffusable positive regulatory element. The srl genes have been ordered relative to the cysC, recA, and alaS genes by two- and three-factor P1kc crosses. The order, cysC...srlD-srlA-srlC-recA-alaS, has been obtained. The srlA and srlD genes comprise an operon with srlD operator distal. From the secondary site lysogen, it has been possible to obtain deletion mutants of this region that are sensitive to ultraviolet light and are recombination deficient. Genetic evidence suggests that these deletions extend from srl into the recA gene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Uptake of fructose by the sorbitol phosphotransferase of Escherichia coli K12. J Gen Microbiol. 1976 Oct;96(2):383–391. doi: 10.1099/00221287-96-2-383. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Estein W. Isolation and characterization of specialized transducing bacteriophages for the recA gene of Escherichia coli. Virology. 1977 Mar;77(1):306–318. doi: 10.1016/0042-6822(77)90427-5. [DOI] [PubMed] [Google Scholar]
- McEntee K., Hesse J. E., Epstein W. Identification and radiochemical purification of the recA protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3979–3983. doi: 10.1073/pnas.73.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K. Specialized transduction of recA by bacteriophage lambda. Virology. 1976 Mar;70(1):221–222. doi: 10.1016/0042-6822(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Ruffler D., Buckel P., Piepersberg W., Böck A. Alanyl-tRNA synthetase of Escherichia coli: genetic analysis of the structural gene and of suppressor mutations. Mol Gen Genet. 1974;134(4):313–323. doi: 10.1007/BF00337466. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]


