Abstract
These studies sought to determine if neurons in the estrogen receptor-α knockout (ERαKO) mouse brain concentrated 16α-[125I]iodo-11β-methoxy-17β-estradiol (125I-estrogen), and if so, whether estrogen binding augmented the expression of progesterone receptor (PR) mRNA. Mice were injected with 125I-estrogen and cryostat sections thaw mounted onto emulsion-coated slides. After 30–90 days of exposure, cells with a nuclear uptake and retention of 125I-estrogen were observed in a number of ERαKO mouse brain regions including the preoptic nucleus and arcuate nucleus of the hypothalamus, bed nucleus of the stria terminalis, and amygdala, although the number of labeled cells and intensity of nuclear concentration was markedly attenuated when compared with wild-type littermates. Competition studies with excess 17β-estradiol, diethylstilbestrol, or moxestrol, but not with R5020 or dihydrotestosterone, prevented the nuclear concentration of 125I-estrogen. To determine if the low level of estrogen binding was capable of regulating gene expression, in situ hybridization was used to evaluate PR mRNA in the brain. ERαKO and wild-type mice were ovariectomized and treated with vehicle or 17β-estradiol, and brains were sectioned and hybridized with a PR cRNA probe. Analysis of hybridization signal revealed a similar, low level of PR mRNA in ovariectomized wild-type and homozygous mice, and a marked increase in expression after treatment of ovariectomized animals with 17β-estradiol, with the level of hybridization signal being significantly higher in wild-type animals when compared with ERαKO mice. The results demonstrate that estrogen binds in the ERαKO brain and is capable of modulating PR gene expression, thus supporting the presence and functionality of a nonclassical estrogen receptor.
Estrogen is known to act in discrete regions of the female brain to regulate gamete production, proceptive and receptive behaviors, and other aspects of reproduction. Estrogens appear to regulate these physiological events by binding to the intracellular estrogen receptor (ER). The ligand-bound receptor then interacts with an estrogen response element on DNA and thereby modulates the transcription of specific genes. ER and its mRNA have been detected in brain regions where estrogen plays a central role in the regulation of events essential for procreation (1, 2).
Recently, a transgenic mouse was constructed that lacked a functional ER-α (3). Initial analysis revealed that ER-α knockout (ERαKO) mice were infertile, failing to show reproductive behavior or a normal estrous cycle (3). Interestingly, in vitro ligand binding studies revealed a 5% residual estrogen binding in the ERαKO uterus (3), although Western blot analysis was unable to detect ER protein (4). Immunocytochemical studies with the ERαKO mice were also unable to detect ER immunoreactivity in the brain (5). In spite of these findings, our laboratory has shown that several neurotransmitter and hormone receptor systems, thought to be dependent on estrogen, were not dramatically attenuated in the ERαKO hypothalamus (P.J.S. and I.M., unpublished observations). These observations suggested that some genes that are regulated by estrogen in the wild-type animal were still sensitive to changes in estrogen in the ERαKO mouse brain.
By using high resolution steroid autoradiography with iodinated estrogen, this study has demonstrated the presence of estrogen target cells in the wild-type and ERαKO brain. Moreover, in situ hybridization studies have shown that estrogen regulates progesterone receptor (PR) gene expression, a gene known to be dramatically up-regulated by estrogen (6), in the preoptic nucleus of the ERαKO mouse hypothalamus.
MATERIALS AND METHODS
C57BL/6J ER-α-disrupted mice (3) were genotyped by using PCR analysis of tail samples to assess the presence of the neomycin resistance and/or ER-α mRNAs (4). Pups exhibiting only the neomycin resistance mRNA were considered homozygous mice, whereas animals that express only the ER-α were wild types. The studies described in this paper were reviewed and approved by the Radnor Animal Care and Use Committee at Wyeth–Ayerst Research.
On postnatal day 18, ERαKO (n = 5) and wild-type (n = 3) mice were ovariectomized (ovx) and returned to their mothers. Seven days after surgery, the ovx mice were s.c. injected in the dorsal cervical region with 0.2 μg/100 g body weight of 16α-[125I]iodo-11β-methoxy-17β-estradiol [125I-estrogen; see ref. 7; specific activity 2,200 Ci/mM, 1 Ci = 37 GBq; purchased from R. Hochberg (Yale University, New Haven, CT)] dissolved in 50% dimethyl sulfoxide (DMSO)/40% PBS/10% ethanol. For competition studies, 1 hr before injecting 125I-estrogen, additional ovx postnatal mice were each injected with 20 μg of an estrogenic compound, 17β-estradiol (Sigma; n = 4), diethylstilbestrol (Sigma; n = 2) or moxestrol (RU2858; a gift from R. Hochberg; n = 2), a synthetic progestin R5020 (NEN; n = 2), or the androgen dihydrotestosterone (Sigma; n = 2), dissolved as described above. Two hours after injection of 125I-estrogen, the brains were collected, frozen, and 10 μm coronal cryostat sections thaw mounted onto emulsion-coated slides (Kodak NTB3, Eastman Kodak). Section-mounted slides were exposed at −30°C in light-tight desiccator slide boxes. After 3–90 days of exposure, the slides were developed and coverslipped. Brain autoradiograms were scanned at low magnification with a light microscope to determine the regional distribution of estrogen target cells in the forebrain regions of wild-type and ERαKO mice. In an attempt to assess the nuclear uptake and retention of radiolabeled estrogen in ERαKO and wild-type brain, sections exposed for a short period of time (10 days) were viewed with high magnification light microscopy. Cells in the preoptic nucleus were further evaluated to determine the number of silver grains concentrated over the nucleus of individual neurons. The results from two sequential sections per animal were averaged and two-way analysis of variance was used to test for differences in the number of silver grains concentrated over cells. It should be noted that in vivo steroid autoradiography is not a quantifiable technique, and therefore, these values are merely an indication of the differences in the degree of nuclear uptake and retention of radiolabeled ligand. Details concerning slide and tissue preparation, freezing, and the use of iodinated steroids for in vivo high resolution autoradiography have been described (8).
PR gene expression was subsequently evaluated in the preoptic nucleus of ERαKO mice with in situ hybridization. Sixty-day-old female wild-type and ERαKO mice were ovx for 5 days and then treated with 5 μg of 17β-estradiol dissolved in 10% DMSO/10% ethanol/80% saline or vehicle alone (n = 4 per group). Six hours after estradiol injection, the ovx animals and five additional intact ERαKO mice were euthanized, their brains frozen, and 20 μm coronal cryostat sections collected on Silane-coated slides (Histology Control Systems, Glen Head, NY). A fragment (bases 2177–2992) of the rat PR cDNA was amplified by using PCR and the rPR-2 plasmid [see ref. 9; a gift from Park-Sarge (University of Kentucky, Lexington)] and subcloned into a pBluescript plasmid (Stratagene). The PR-815 plasmid was linearized with HindIII (sense; control) or BamHI (antisense) and used to generate [35S]UTP-labeled cRNA probes for in situ hybridization. Processed section-mounted slides were hybridized with 100–200 μl of an antisense or sense (control) riboprobe (6 × 106 dpm per slide) −50% formamide hybridization mix and incubated overnight at 55°C in an open-air humidified slide chamber. The slides were washed, dehydrated, and apposed to BioMax (BMR-1; Eastman Kodak) x-ray film for 4 days and then dipped in NTB2 nuclear emulsion (Eastman Kodak). The slides were exposed for 4–6 weeks, photographically processed, stained, and coverslipped. The slides from all animals were hybridized, washed, exposed, and photographically processed together to eliminate differences due to interassay variations in conditions. Details of the in situ hybridization method have been reported previously (10).
The medial preoptic nucleus (Fig. 1) was selected for statistical evaluation because the level of PR mRNA in this brain region is highly sensitive to estrogenic action and has been well characterized (6, 12). Relative optical density measurements of PR hybridization signal were obtained from film autoradiograms with a computer-based image analysis system (C-Imaging, Pittsburgh). The results from two sequential sections per animal were averaged and two-way analysis of variance was used to test for differences in the level of PR mRNA. The same size sampling box was used for each treatment group to ensure consistent sampling among groups. The image analysis system was calibrated with optical density linear standards (calibration step table no. 809ST601; Eastman Kodak) before image analysis to ensure that all semi-quantitative measurements were within the linear range. The numerical values are reported as the mean ± SE, and all statements of difference imply that P < 0.05.
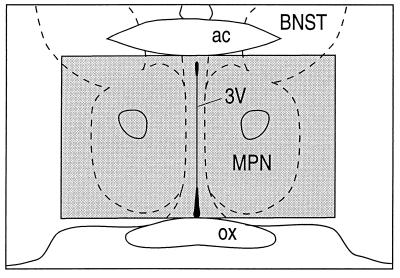
Figure 1.
Schematic drawing depicting the region of the preoptic nucleus where PR mRNA was evaluated (shaded box). The drawing was modified from the atlas of Paxinos and Watson (11). 3V, third ventricle; ac, anterior commissure; BNST, bed nucleus of the stria terminalis; MPN, medial preoptic nucleus; ox, optic chiasm.
RESULTS
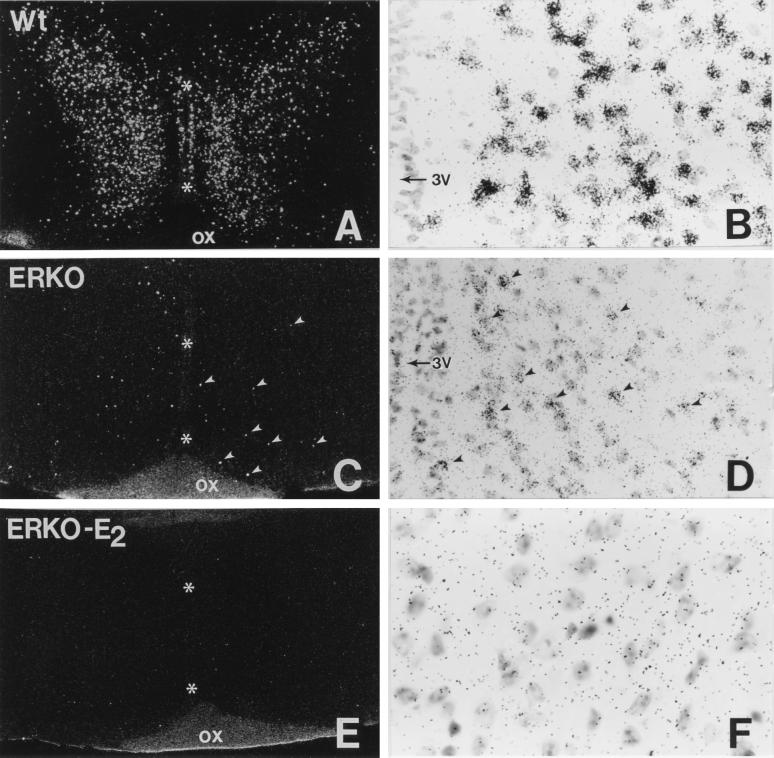
The distribution of estrogen binding sites observed in wild-type animals was in good agreement with previous autoradiographic studies in rodents (8, 13–18). Labeled cells were concentrated in the medial preoptic nucleus (Fig. 2 A and B), ventromedial nucleus and arcuate nucleus of the hypothalamus, bed nucleus of the stria terminalis, and amygdala, whereas additional labeled cells were seen in the cerebral cortex, hippocampus, and other brain regions. In ERαKO mice, weak estrogen binding was also detected in the medial preoptic nucleus (Fig. 2 C and D), bed nucleus of the stria terminalis, arcuate nucleus, and medial amygdaloid nucleus, although the degree of labeling in the arcuate nucleus was attenuated when compared with other brain regions. Surprisingly, no binding was seen in the ventromedial hypothalamic nucleus of ERαKO mice. In addition to regional differences in 125I-estrogen binding seen in the wild-type and ERαKO brain, the number of labeled cells and the number of silver grains concentrated over each cell nucleus were markedly reduced in ERαKO mice (Fig. 2 C and D, Table 1) as compared with wild-type animals (Fig. 2 A and B, Table 1). Although this technique does not enable one to determine the exact number of estrogen binding sites in a cell, the evaluation of silver grains concentrated over cells in the preoptic nucleus indicates that ovx wild-type animals have significantly more silver grains per cell than ovx ERαKO mice (82.3 ± 6 vs. 9.6 ± 1). Competition studies with 17β-estradiol (Fig. 2 E and F, Table 1), diethylstilbestrol, or moxestrol, but not with a progestin or an androgen, prevented the nuclear uptake and retention of 125I-estrogen in the wild-type and ERαKO (0.3 ± 0.2) brains.
Figure 2.
Autoradiographic images of the medial preoptic nucleus in the wild-type (A and B) and ERαKO (C–F) female mouse brain 2 hr after subcutaneous injection of 125I-estrogen. Note the attenuated number of labeled cells and concentration of silver grains over each neuron in the ERαKO preoptic nucleus after a comparable exposure time (C and D). The binding of 125I-estrogen in the ERαKO preoptic nucleus was specific for an estrogen binding site because the concentration of silver grains over cells was eliminated when animals were treated with unlabeled 17β-estradiol prior to the injection of the radiolabeled estrogen (E and F). Arrowheads indicate some of the labeled cells in C and D and asterisks indicate the third ventricle of the hypothalamus (A, C, and E). 3V, third ventricle; ox, optic chiasm. Autoradiographic exposure time is 40 days.
Table 1.
Number of silver grains concentrated over cells in the preoptic nucleus
| Genotype | Mean | SE |
|---|---|---|
| Wild-type ovx | 82.3 | 6.1*** |
| ERαKO ovx | 9.6 | 1.0 |
| ERαKO ovx competition with E2 | 0.3 | 0.2* |
Statistically significant difference from ERαKO ovx: ∗, P < 0.05; ∗∗∗, P < 0.001.
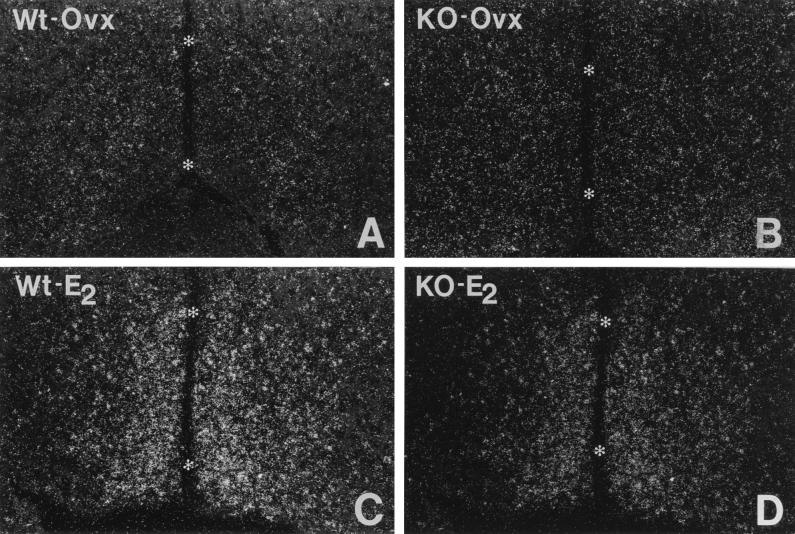
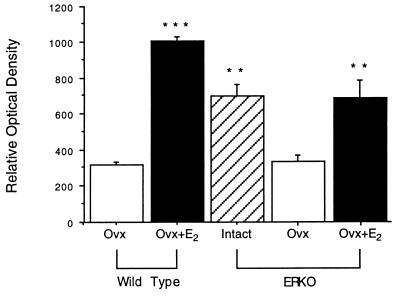
To evaluate the functional capacity of estrogen in the ERαKO mouse, PR mRNA regulation was assessed in the preoptic nucleus of the hypothalamus. When ovx wild-type mice were injected with 17β-estradiol, a significant increase in the level of PR mRNA was observed in the preoptic nucleus (Figs. 3C and 4) when compared with vehicle-treated ovx animals (Figs. 3A and 4). Although the level of PR mRNA detected in intact ERαKO females was attenuated when compared with estrogen-treated ovx wild-type animals, it was significantly higher than ovx wild-type mice (Fig. 4). When ERαKO mice were ovx (Figs. 3B and 4), the level of PR mRNA decreased significantly to a level comparable to wild-type ovx animals (Fig. 4). The treatment of ovx ERαKO females with estradiol (Figs. 3D and 4) markedly augmented PR gene expression to a level comparable to intact ERαKO mice.
Figure 3.
PR mRNA in the medial preoptic nucleus of wild-type (A and C) and ERαKO (B and D) female mice by in situ hybridization. Note the marked increase in hybridization signal when ovx wild-type and ERαKO mice (A and B) are treated with estradiol for 6 hr (C and D). Asterisks indicate the third ventricle.
Figure 4.
The hybridization signal for PR mRNA in the medial preoptic nucleus detected with in situ hybridization. Note the dramatic increase in hybridization signal when ovx wild-type mice were treated with estradiol. Similarly, the hybridization signal seen in intact ERαKO animals was attenuated when mice were ovx, but augmented to intact levels when ovx animals were treated with estradiol. Statistical significance is indicated as follows: ∗∗, P < 0.01; ∗∗∗, P < 0.001.
DISCUSSION
These studies have demonstrated that neurons in the ERαKO mouse brain, including cells in the preoptic nucleus of the hypothalamus, have a nuclear uptake and retention of radiolabeled estrogen and shown that 17β-estradiol binding regulates the expression of PR mRNA in this brain region. This study, as well as previous studies with rodents (8, 13–18), have detected estrogen binding in the preoptic nucleus, arcuate nucleus and ventromedial nucleus of the hypothalamus, bed nucleus of the stria terminalis, amygdala, cerebral cortex, and hippocampus. In the ERαKO mouse brain, specific nuclear uptake and retention of radiolabeled estrogen was also detected in the preoptic nucleus, arcuate nucleus, bed nucleus of the stria terminalis, and amygdala, although the number of labeled cells and intensity of nuclear concentration of radiolabeled estrogen in the ERαKO brain were markedly attenuated when compared with wild-type littermates. Surprisingly, this study was unable to detect specific binding in the cortex, hippocampus, or ventromedial hypothalamic nucleus of ERαKO mice and the degree of labeling in the arcuate nucleus was markedly attenuated when compared with other brain regions. Because the binding of 125I-estrogen in the ERαKO brain was competed off with unlabeled 17β-estradiol, diethylstilbestrol, or moxestrol, but not with the progestin R5020 or dihydrotestosterone, the nuclear concentration of radiolabeled estrogen appears to be specific for an estrogen binding site.
The expression of PR mRNA is known to be dramatically and rapidly up-regulated in the rodent preoptic nucleus after the injection of a small dose of 17β-estradiol (6). To determine if the low level of estrogen binding seen in the ERαKO brain was capable of regulating gene expression, PR mRNA was evaluated in the preoptic nucleus of ERαKO and wild-type mice. Analysis of hybridization signal revealed a similar, low level of PR mRNA in ovx, wild-type, and homozygous mice, and a marked increase in PR mRNA expression after treatment of ovx animals with 17β-estradiol. These observations demonstrated that 17β-estradiol was capable of modulating the expression of PR mRNA in the brain of ER-α-disrupted mice.
Although these studies have definitively shown that radiolabeled estrogen is concentrated in the nuclei of certain neuronal populations and competed off with natural and synthetic estrogens, they have not elucidated what the nature of the binding site is in the ERαKO brain. The most plausible explanation for the residual binding seen in brain is the interaction of 125I-estrogen with ER-β. ER-β, a novel member of the steroid receptor superfamily with a high degree of sequence homology with the classical ER (now denoted ER-α) and specific binding affinity for 17β-estradiol, was recently cloned by Kuiper and colleagues (19). Analysis of the distribution of ER-β mRNA in the rat brain with in situ hybridization revealed that ER-β mRNA was concentrated in the preoptic nucleus, supraoptic nucleus, and paraventricular nucleus of the hypothalamus, bed nucleus of the stria terminalis, and medial amygdala (20). Weak ER-β hybridization signal was also seen in the arcuate nucleus, whereas no specific signal was detected in the ventromedial hypothalamic nucleus (20). A comparison of the binding of radiolabeled estrogen in the ERαKO brain reported here, and the distribution of ER-β mRNA in the rat (20) and adult ERαKO mouse (J. F. Couse, J.-Å. Gustafsson, and K.S.K., unpublished observations; P.J.S. and I.M., unpublished observations) suggests that the residual 125I-estrogen binding may be due to the interaction with ER-β, although the distribution of ER-β mRNA in the 25-day-old mouse brain is currently unknown. Moreover, the absence of ER-α immunoreactivity in the ERαKO brain by using ER antibodies (5), which do not recognize ER-β (21), provides additional evidence for the interaction of 125I-estrogen with ER-β. Therefore, estrogen binding to ER-β in certain regions, such as the preoptic nucleus of the ERαKO brain may maintain estrogen-regulated genes including PR. In contrast, the biological activity of estrogen in regions of the mouse brain that only express ER-α would be attenuated or eliminated in the ERαKO brain. For example, the ventromedial hypothalamic nucleus, a critical region for hormonal regulation of reproductive behavior, expresses only ER-α. Thus, the observation that ERαKO mice have deficiencies in lordosis and maternal behavior is not surprising (22).
Although these data suggest that 125I-estrogen is binding with ER-β in the ERαKO mouse brain, it is intriguing that the 125I-estrogen binding is very weak considering the similar levels of ER-β mRNA in ERαKO and wild-type mice (J. F. Couse, J.-Å. Gustafsson, and K.R.K., unpublished observations; P.J.S. and I.M., unpublished observations). In addition, the regulation of PR mRNA by 17β-estradiol is much greater than what is expected. This suggests that the marked reduction in labeling seen in the ERαKO mouse brain may be due to the weak affinity of 125I-estrogen for ER-β. Future studies with estrogenic compounds that have a high specific activity and a better affinity for ER-β are needed to resolve this discrepancy.
An alternative explanation for the residual binding seen in the ERαKO brain is a splicing variant of ER-α. Splicing variants are thought to arise when nonsense codons are encountered in an exon and are “spliced out” (23) or when the neomycin resistance gene has been inserted into the exon by gene targeting (24). Mutant ER isoforms lacking different exons have been detected in primary tumors and cancer cell lines (25, 26). An isoform lacking exon 4 has also been found in normal rat brain, although since exon 4 encodes a portion of the ligand binding domain, it is unlikely that this protein binds estrogen (27). Couse and coworkers have isolated and characterized two splicing variants from the ERαKO uterus that have resulted from a partial or full “splice out” of the neomycin resistance gene (4). The splicing event that generates one of the variants (E2) also produces a reading frame shift that introduces two premature stop codons (4). The other variant (E1) remains in-frame and appears to be present in the ERαKO uterus, although the transcriptional activity of this receptor variant is known to be significantly attenuated (4). Although unlikely, these observations suggest that a splicing variant of ER may exist in the ERαKO mouse brain and account for the residual binding and regulation of PR mRNA. A final possibility is that additional novel nuclear receptors for estrogen may exist in the brain. Additional studies are required to resolve these issues.
In these studies, we have demonstrated the presence of estrogen binding in the brain of ER-α-disrupted mice and shown that estradiol is capable of regulating the expression of PR mRNA. Based on these observations, estrogen appears to regulate some genes in the developing and adult ERαKO brain via a nonclassical ER.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ERαKO, estrogen receptor-α knockout; PR, progesterone receptor; 125I-estrogen, 16α-[125I]iodo-11β-methoxy-17β-estradiol; ER, estrogen receptor; ovx, ovariectomized; ER-α, estrogen receptor-α; ER-β, estrogen receptor-β.
References
- 1.Sodersten P. In: Actions of Progesterone on the Brain. Ganten D, Pfaff D W, editors. Berlin: Springer; 1985. pp. 141–173. [Google Scholar]
- 2.Blaustein J D, Olster D H. In: Advances in Comparative Environmental Physiology. Balthazart J, editor. Berlin: Springer; 1989. pp. 31–104. [Google Scholar]
- 3.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shughrue, P. J., Lane, M. V. & Merchenthaler, I. (1997) Endocrinology, in press. [DOI] [PubMed]
- 7.Zielinski J E, Yabuki H, Pahuja S L, Larner J M, Hochberg R B. Endocrinology. 1986;119:130–139. doi: 10.1210/endo-119-1-130. [DOI] [PubMed] [Google Scholar]
- 8.Shughrue P J, Stumpf W E. In: Autoradiography and Correlative Imaging. Stumpf W E, Soloman H F, editors. New York: Academic; 1995. pp. 129–149. [Google Scholar]
- 9.Park O-K, Mayo K E. Mol Endocrinol. 1991;5:967–978. doi: 10.1210/mend-5-7-967. [DOI] [PubMed] [Google Scholar]
- 10.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- 12.Simerly R B, Carr A M, Zee M C, Lorand D. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 13.Sibug R M, Stumpf W E, Shughrue P J, Hochberg R B, Drews U. Dev Brain Res. 1991;61:11–22. doi: 10.1016/0165-3806(91)90109-v. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan P J. Brain Res. 1979;178:201–206. doi: 10.1016/0006-8993(79)90101-x. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach J L, McEwen B S, Toran-Allerand C D, Friedman W J. Dev Brain Res. 1983;11:7–18. doi: 10.1016/0165-3806(83)90197-9. [DOI] [PubMed] [Google Scholar]
- 16.Shughrue P J, Stumpf W E, MacLusky N J, Zielinski J E, Hochberg R B. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 17.Stumpf W E, Sar M. In: Anatomical Neuroendocrinology. Stumpf W E, Grant L D, editors. New York: Karger; 1974. pp. 82–103. [Google Scholar]
- 18.Pfaff D, Keiner M. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shughrue P J, Komm B, Merchenthaler I. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper G G J M, Carlsson B, Grandien K A J, Enmark E, Haggblad J, Nilsson S, Gustafsson J-Å. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S, Taylor J A, Lubahn D B, Korach K S, Pfaff D W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 23.Dietz H C, Valle D, Francomano C A, Kendzior R J, Jr, Pyeritz R E, Cutting G R. Science. 1993;259:680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- 24.Luetteke N C, Qiu T H, Peiffer R L, Oliver P, Smithies O, Lee D C. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 25.McGuire W L, Chamness G C, Fuqua S A W. Mol Endocrinol. 1991;5:1571–1577. doi: 10.1210/mend-5-11-1571. [DOI] [PubMed] [Google Scholar]
- 26.Koh E H, Ro J, Wildrick D M, Hortobagyi G N, Blick M. Anticancer Res. 1989;9:1841–1845. [PubMed] [Google Scholar]
- 27.Skipper J K, Young L J, Bergeron J M, Tetzlaff M T, Osborn C T, Crews D. Proc Natl Acad Sci USA. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]