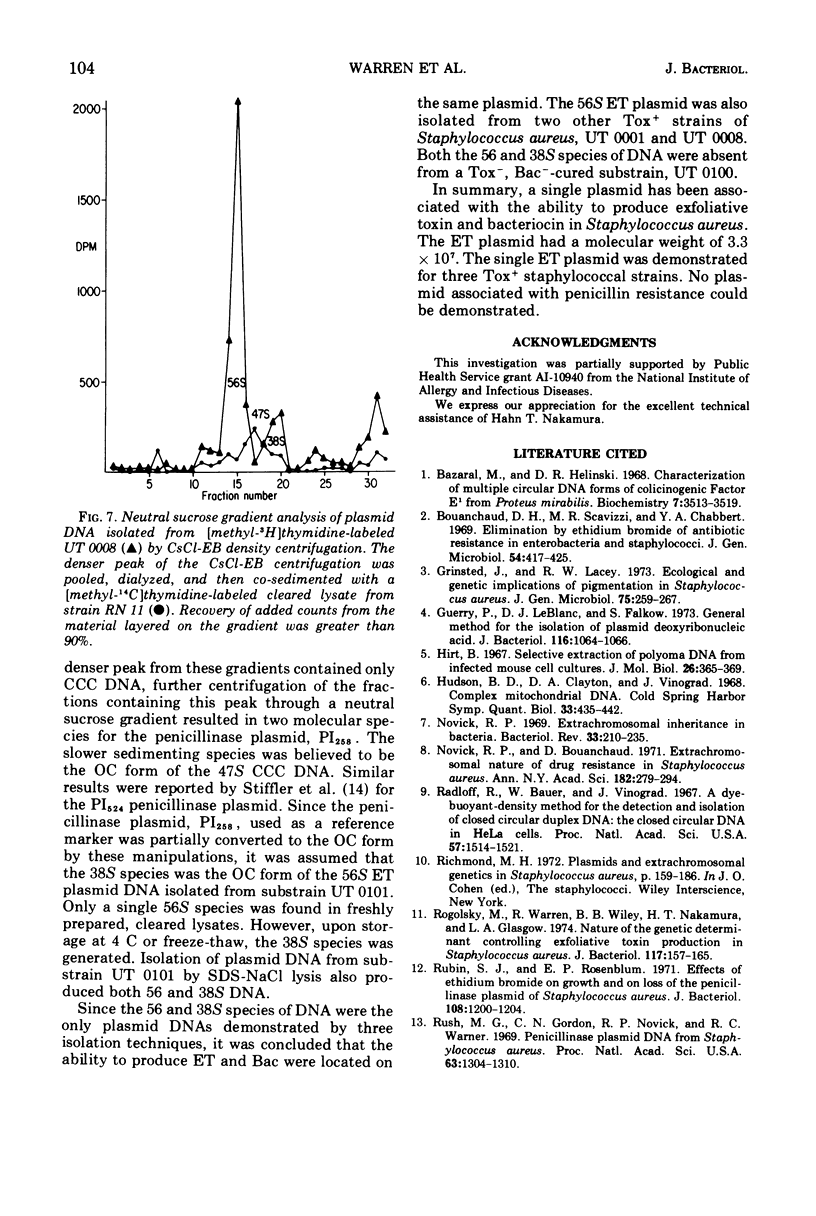
Abstract
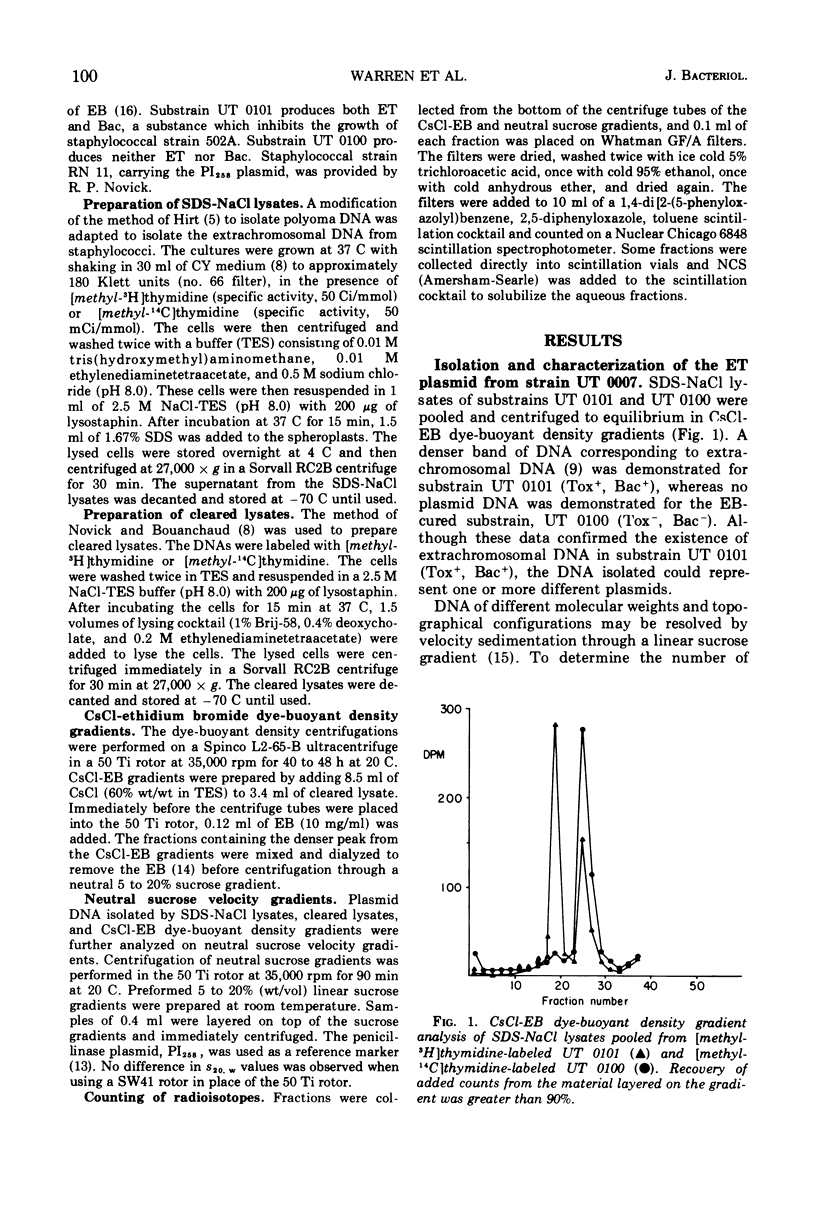
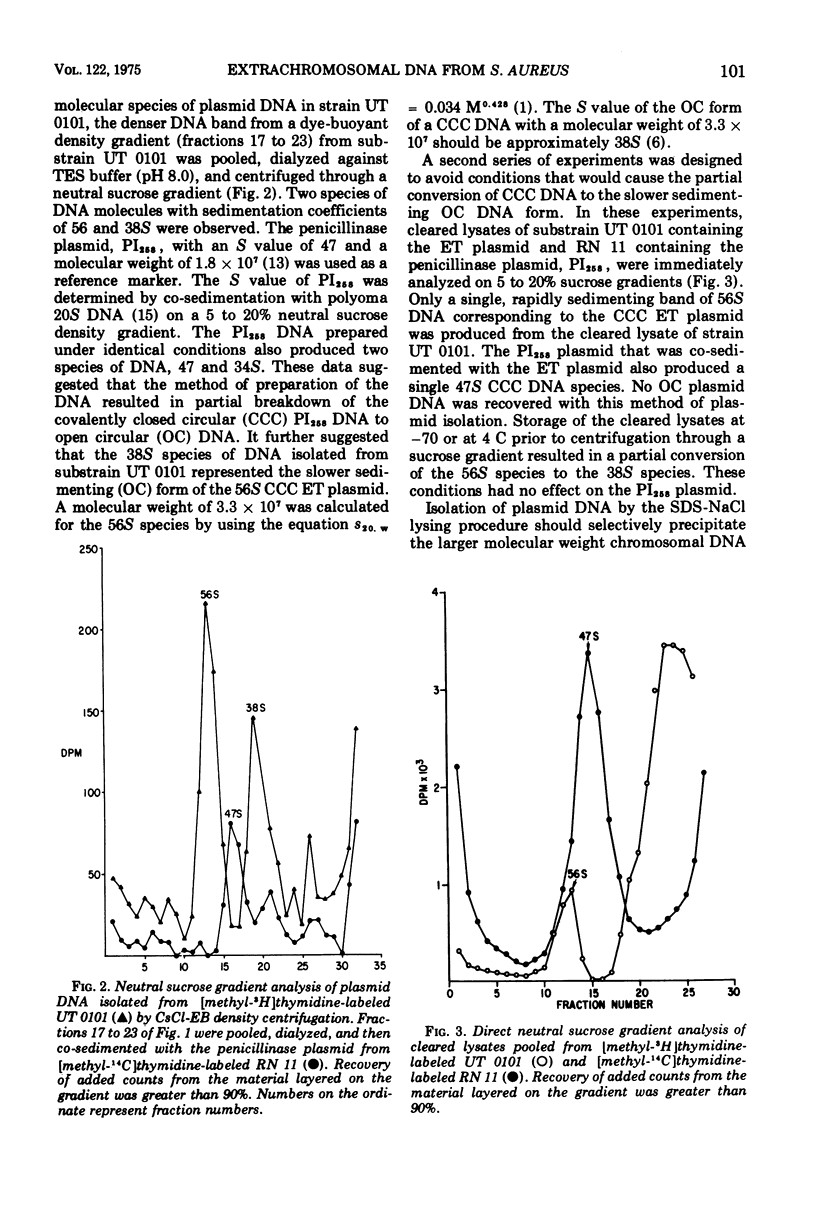
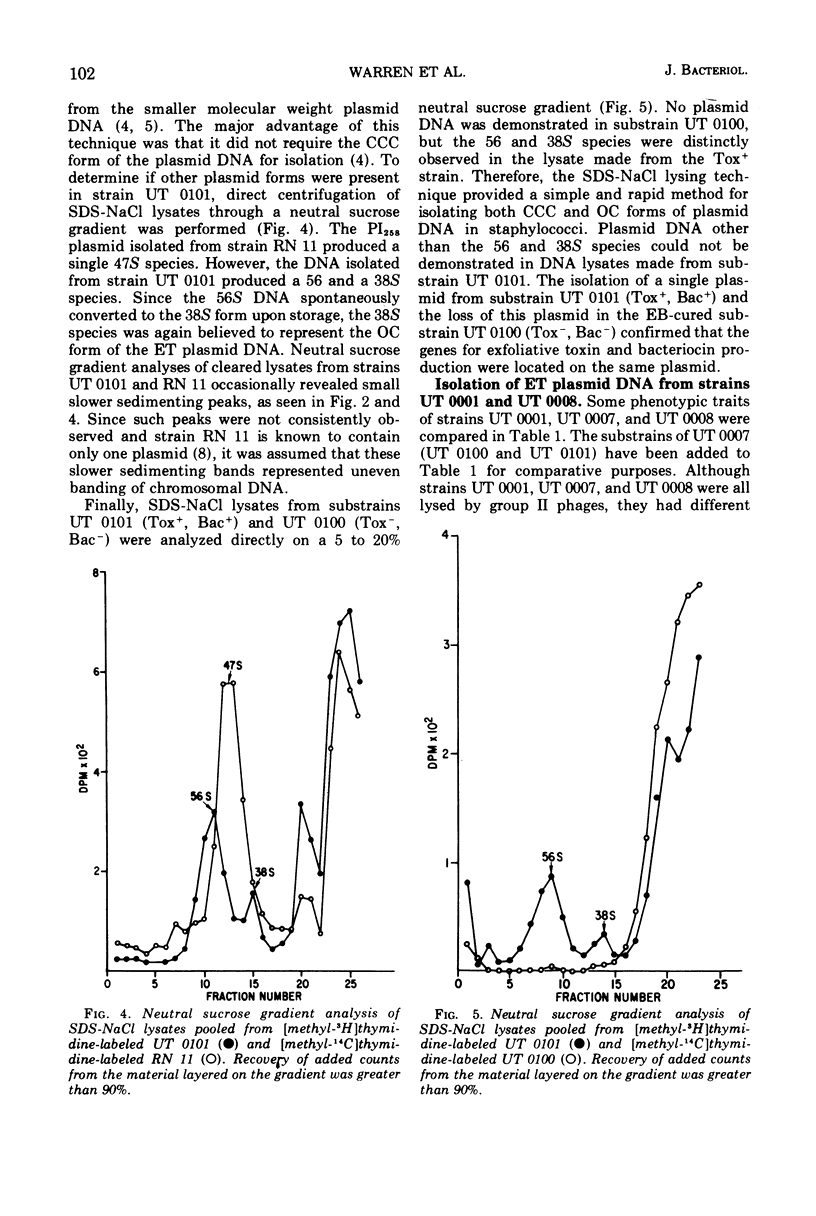
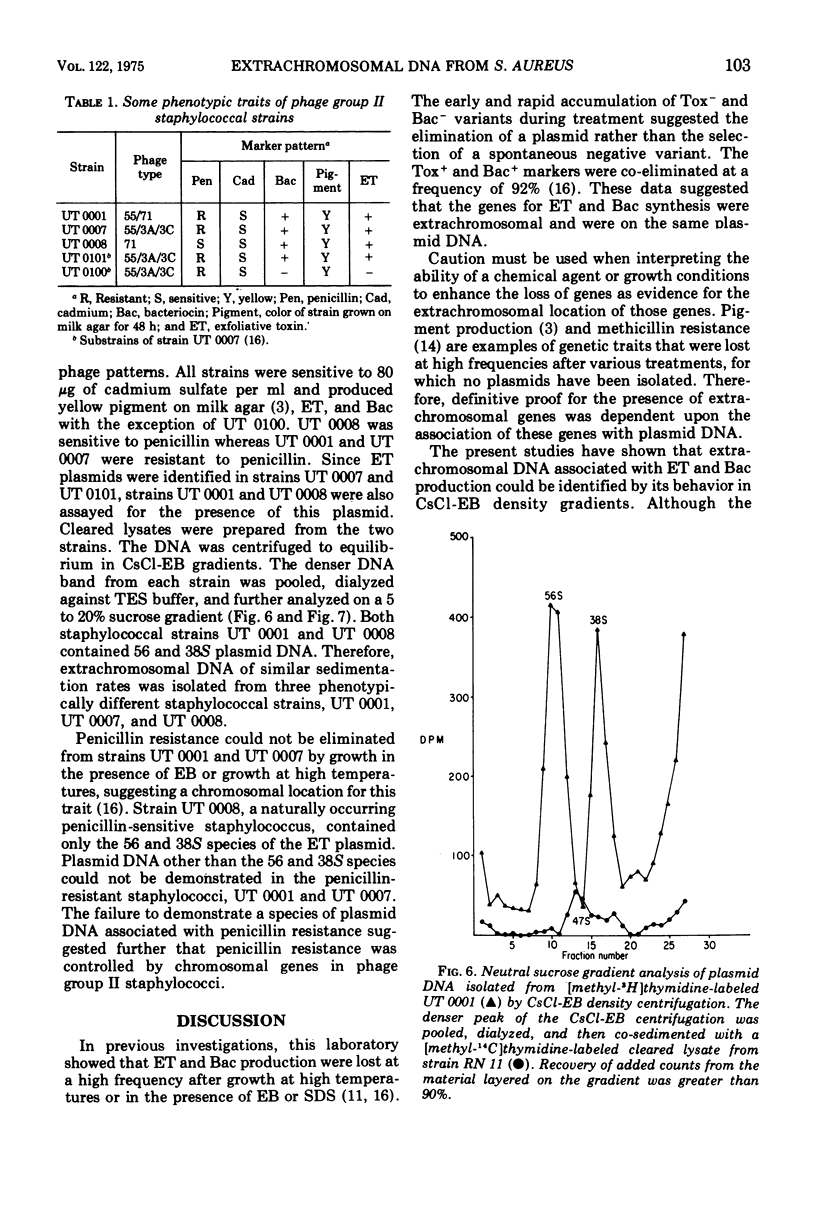
The ability of phage group II staphylococcal strain UT 0101 to produce exfoliative toxin and bacteriocin could be eliminated at a high frequency after growth at high temperatures or in the presence of ethidium bromide or sodium dodecyl sulfate. Extrachromosomal deoxyribonucleic acid, associated with the genes for exfoliative toxin and bacteriocin production, was isolated from strain UT 0101 but was absent from an ethidium bromide-cured substrain. The molecular weight of the exfoliative toxin plasmid, determined by co-sedimentation with the penicillinase plasmid, PI258, was 3.3 times 10-7. The 56S covalently closed circular form of the exfoliative toxin plasmid converted to a 38S open circular form after storage or exposure to sodium dodecyl sulfate. Plasmid deoxyribonucleic acid associated with penicillin resistance could not be identified in the penicillin-resistance Tox+ strains, UT 0007 and UT 0001.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Lacey R. W. Ecological and genetic implications of pigmentation in Staphylococcus aureus. J Gen Microbiol. 1973 Apr;75(2):259–267. doi: 10.1099/00221287-75-2-259. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Warren R., Wiley B. B., Nakamura H. T., Glasgow L. A. Nature of the genetic determinant controlling exfoliative toxin production in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):157–165. doi: 10.1128/jb.117.1.157-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S. J., Rosenblum E. D. Effects of ethidium bromide on growth and on loss of the penicillinase plasmid of Staphylococcus aureus. J Bacteriol. 1971 Dec;108(3):1200–1204. doi: 10.1128/jb.108.3.1200-1204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R., Rogolsky M., Wiley B. B., Glasgow L. A. Effect of ethidium bromide on elimination of exfoliative toxin and bacteriocin production in Staphylococcus aureus. J Bacteriol. 1974 Jun;118(3):980–985. doi: 10.1128/jb.118.3.980-985.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


