Abstract
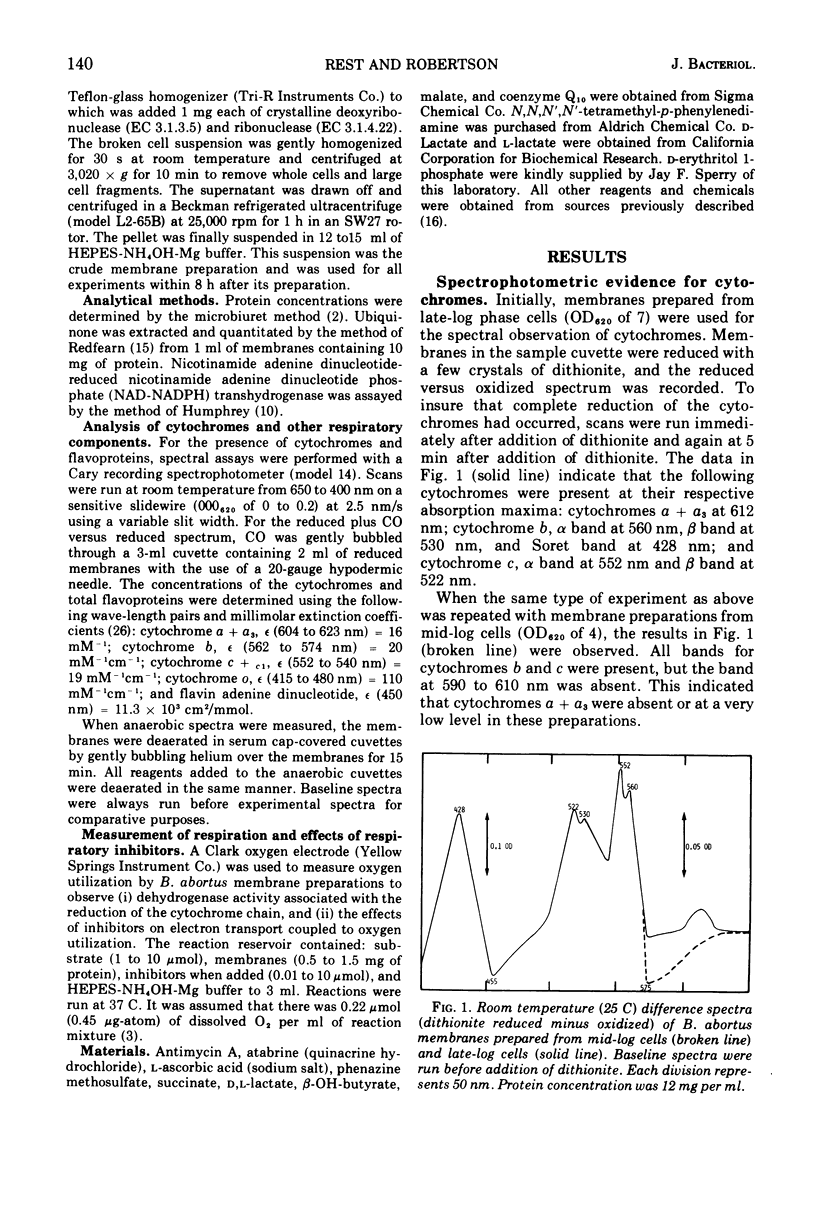
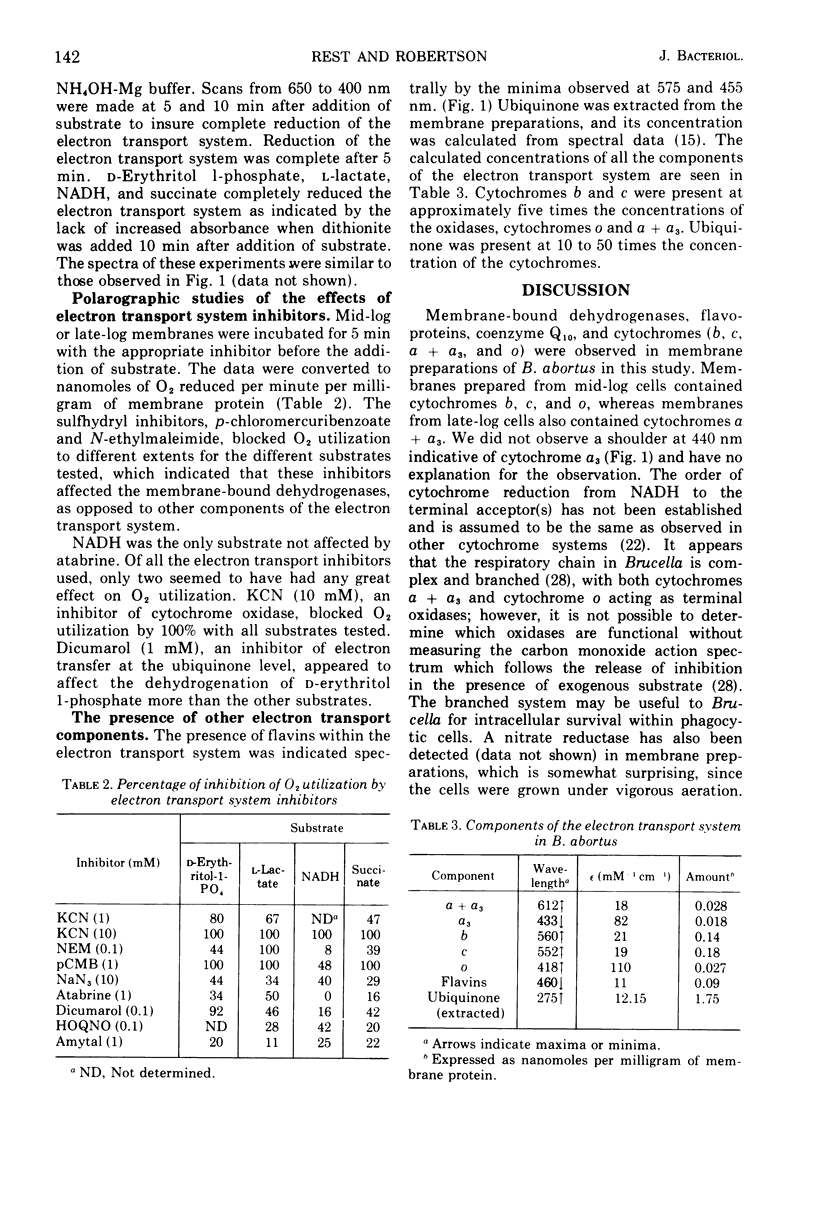
The electron transport system in Brucella abortus has been characterized. Spectral studies of membrane preparations have indicated the presence of cytochromes a + a3 (maxima at 612 nm), cytochrome b (maxima at 560, 530, and 428 nm), cytochrome c (maxima at 552 and 522 nm), cytochrome o (maxima of carbon monoxide complex at 418 nm), and flavoproteins (minimum at 582 and 450 nm). Cytochromes a + a3 appeared only after cells had reached late log phase, possibly due to lowered oxygen tension in the medium. Dehydrogenases were shown to be present for D-erythritol 1-phosphate, L-lactate, reduced nicotinamide adenine dinucleotide, and succinate. All of the above substrates reduced the electron transport chain and at least some of the flavoproteins, indicating similar pathways of electron transport. N-ethylmaleimide, p-chloromercuribenzoate, and KCN were the only electron transport inhibitors that blocked electron transport by 100%. The system seemed to be uniquely resistant to other electron transport inhibitors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- Carr N. G., Exell G. Ubiquinone concentrations in athiorhodaceae grown under various environmental conditions. Biochem J. 1965 Sep;96(3):688–692. doi: 10.1042/bj0960688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. The nature of electron transfer and energy coupling reactions. FEBS Lett. 1972 Jun 1;23(1):3–20. doi: 10.1016/0014-5793(72)80272-2. [DOI] [PubMed] [Google Scholar]
- Felix J. A., Lundgren D. G. Electron transport system associated with membranes of Bacillus cereus during vegetative growth and sporulation. J Bacteriol. 1973 Aug;115(2):552–559. doi: 10.1128/jb.115.2.552-559.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY G. F. The distribution and properties of transhydrogenase from animal tissues. Biochem J. 1957 Mar;65(3):546–550. doi: 10.1042/bj0650546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Kamen M. D. Bacterial cytochromes. II. Functional aspects. Annu Rev Microbiol. 1970;24:399–428. doi: 10.1146/annurev.mi.24.100170.002151. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- PAGE A. C., Jr, GALE P., WALLICK H., WALTON R. B., McDANIEL L. E., WOODRUFF H. B., FOLKERS K. Coenzyme Q. 17. Isolation of coenzyme Q10 from bacterial fermentation. Arch Biochem Biophys. 1960 Aug;89:318–321. doi: 10.1016/0003-9861(60)90062-x. [DOI] [PubMed] [Google Scholar]
- RAMAN T. S., SHARMA B. V., JAYARAMAN J., RAMASARMA T. BIOSYNTHESIS OF COENZYME Q IN MICROORGANISMS. Arch Biochem Biophys. 1965 Apr;110:75–84. doi: 10.1016/0003-9861(65)90156-6. [DOI] [PubMed] [Google Scholar]
- RICHARDSON M. Cytochrome oxidase in cells of the genus Brucella. J Bacteriol. 1957 Dec;74(6):699–706. doi: 10.1128/jb.74.6.699-706.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Robertson D. C. Glucose transport in Brucella abortus. J Bacteriol. 1974 Apr;118(1):250–258. doi: 10.1128/jb.118.1.250-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsin B., Brodie A. F. Carbon monoxide-binding pigments of Mycobacterium phlei and Escherichia coli. J Biol Chem. 1969 Jun 10;244(11):3101–3104. [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. II. Cell-free studies with B. abortus (S-19). Arch Biochem Biophys. 1968 Sep 20;127(1):445–456. doi: 10.1016/0003-9861(68)90249-x. [DOI] [PubMed] [Google Scholar]
- Schulp J. A., Stouthamer A. H. The influence of oxygen, glucose and nitrate upon the formation of nitrate reductase and the respiratory system in Bacillus licheniformis. J Gen Microbiol. 1970 Dec;64(2):195–203. doi: 10.1099/00221287-64-2-195. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Kehr W. Die "freien" Lipide aus Brucella abortus Bang. Uber die Neutrallipide. Eur J Biochem. 1969 Jun;9(2):167–175. doi: 10.1111/j.1432-1033.1969.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]


