Abstract
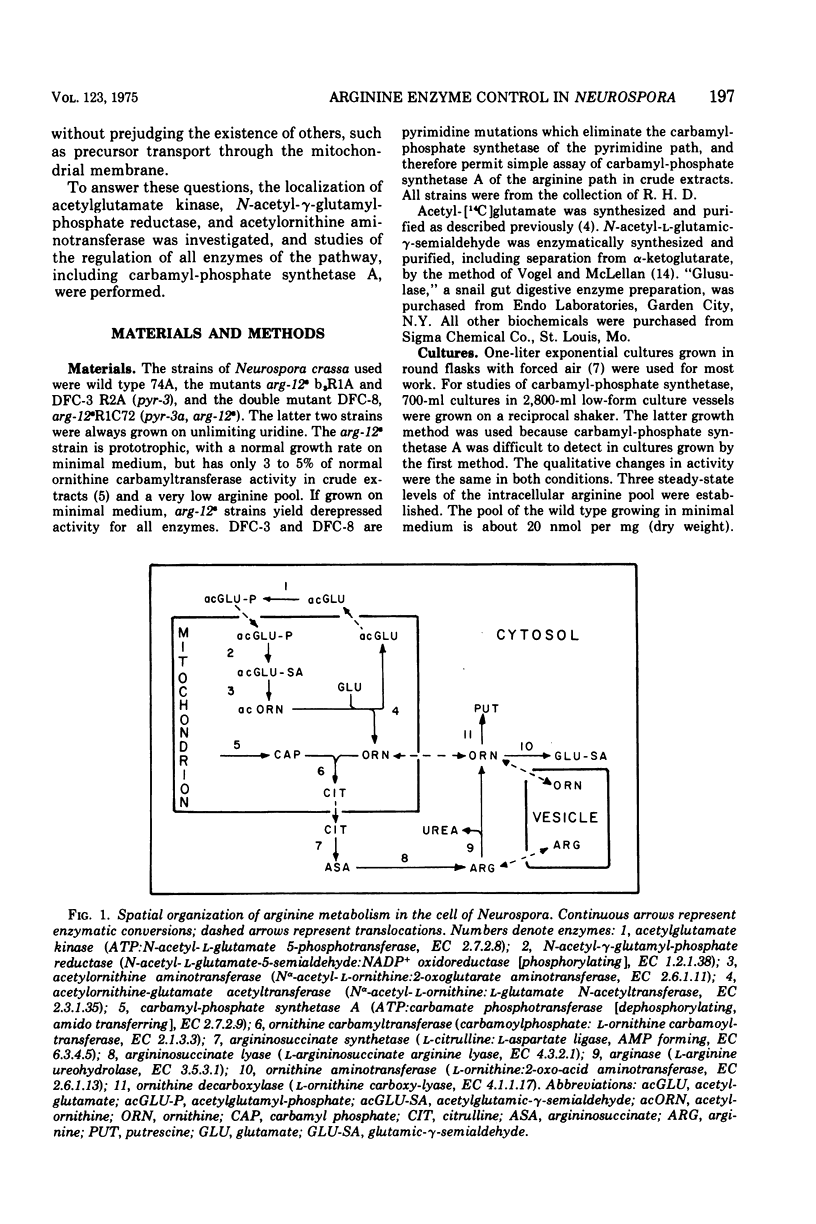
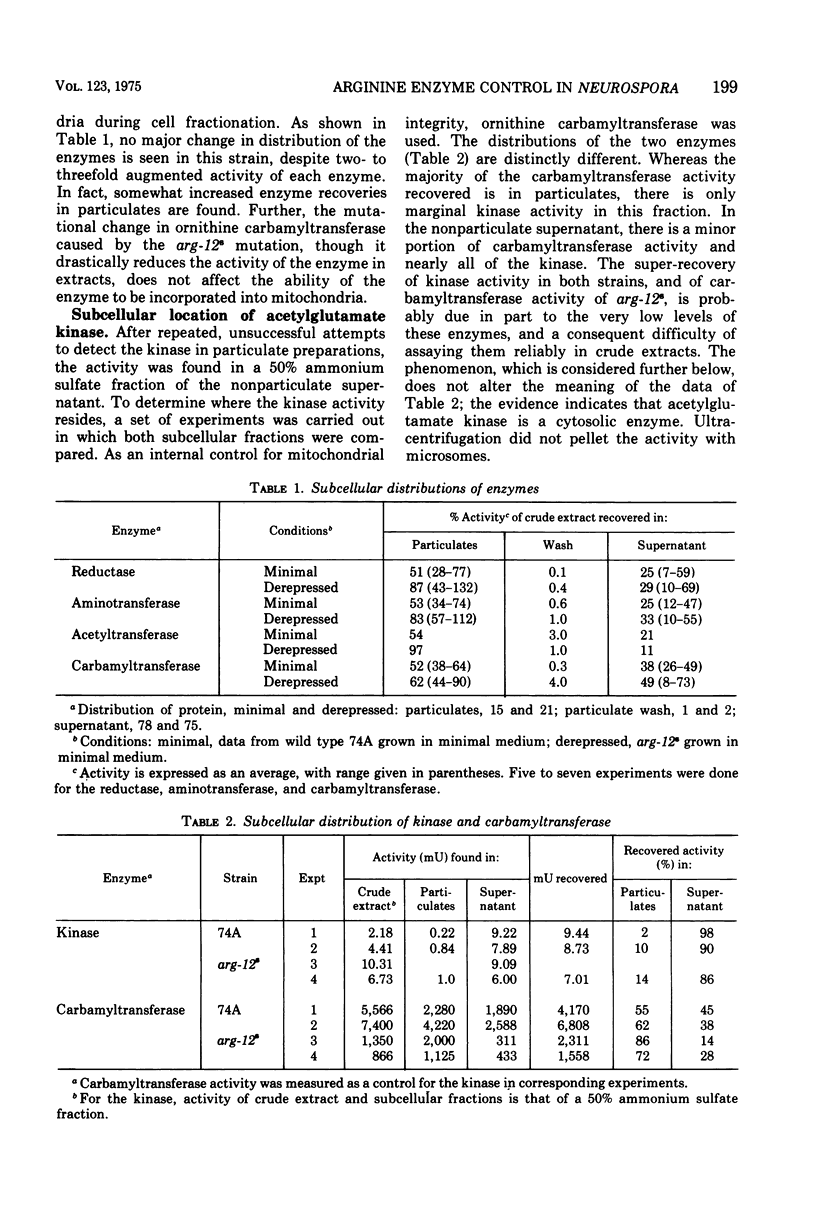
Eight enzymes involved in the conversion of acetylglutamate to arginine in Neurospora crassa were studied. The data indicate that of three enzymes early in the sequence, only the first, acetylglutamate kinase, is a nonorganellar enzyme. The next two, N-acetyl-gamma-glutamyl-phosphate reductase and acetylornithine aminotransferase, are in the mitochondrion, which was previously shown to contain the subsequent enzymes: acetylornithine-glutamate acetyltransferase, ornithine carbamyltransferase, and carbamyl-phosphate synthetase A (arginine specific). The last two enzymes of the pathway, argininosuccinate synthetase and argininosuccinate lyase, were previously shown to be cytosolic. All enzymes but one have low amplitudes or repression. Their levels respond little to arginine excess and are about twofold elevated (threefold for ornithine carbamyltransferase) as a result of arginine limitation in the arg-12-8 strain. No restriction of the incorporation of mitochondrial enzymes into mitochondria could be detected when the levels of these enzymes were elevated. Two enzymes, acetylglutamate kinase and carbamyl-phosphate synthetase A, which initiate the synthesis of the ornithine and guanidino moieties of arginine, respectively, show the lowest specific activities in crude extract. These enzymes display special regulatroy features. Acetylglutamate kinase, which has a typically low amplitude of repression, is subject to feedback inhibition. Carbamyl-phosphate synthetase A is wholly insensitive to arginine or citrulline in vitro or in vivo, but displays a very large amplitude of repression (about 60-fold). It is unique in that it can be almost completely repressed by growth of mycelia in excess arginine. These data suggest that mitochondrial localization may be incompatible with a mechanism of feedback inhibition by a cytosolic effector, arginine. Further, they suggest that the high repressibility of carbamyl-phosphate synthetase A compensates for its feedback insensitivity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Barthelmess I. B., Curtis C. F., Kacser H. Control of the flux to arginine in Neurospora crassa: de-repression of the last three enzymes of the arginine pathway. J Mol Biol. 1974 Aug 5;87(2):303–316. doi: 10.1016/0022-2836(74)90151-x. [DOI] [PubMed] [Google Scholar]
- Bernhardt S. A., Davis R. H. Carbamoyl phosphate compartmentation in Neurospora: histochemical localization of aspartate and ornithine transcarbamoylases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1868–1872. doi: 10.1073/pnas.69.7.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- Hilger F., Culot M., Minet M., Pierard A., Grenson M., Wiame J. M. Studies on the kinetics of the enzyme sequence mediating arginine synthesis in Saccharomyces cerevisiae. J Gen Microbiol. 1973 Mar;75(1):33–41. doi: 10.1099/00221287-75-1-33. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Staub M., Dénes G. Mechanism of arginine biosynthesis in Chlamydomonas reinhardti. I. Purification and properties of ornithine acetyltransferase. Biochim Biophys Acta. 1966 Oct 17;128(1):82–91. doi: 10.1016/0926-6593(66)90144-5. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampler D. E., Fairley J. L. Argininosuccinate synthetase of Neurospora crassa. Arch Biochem Biophys. 1967 Sep;121(3):580–586. doi: 10.1016/0003-9861(67)90041-0. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]


