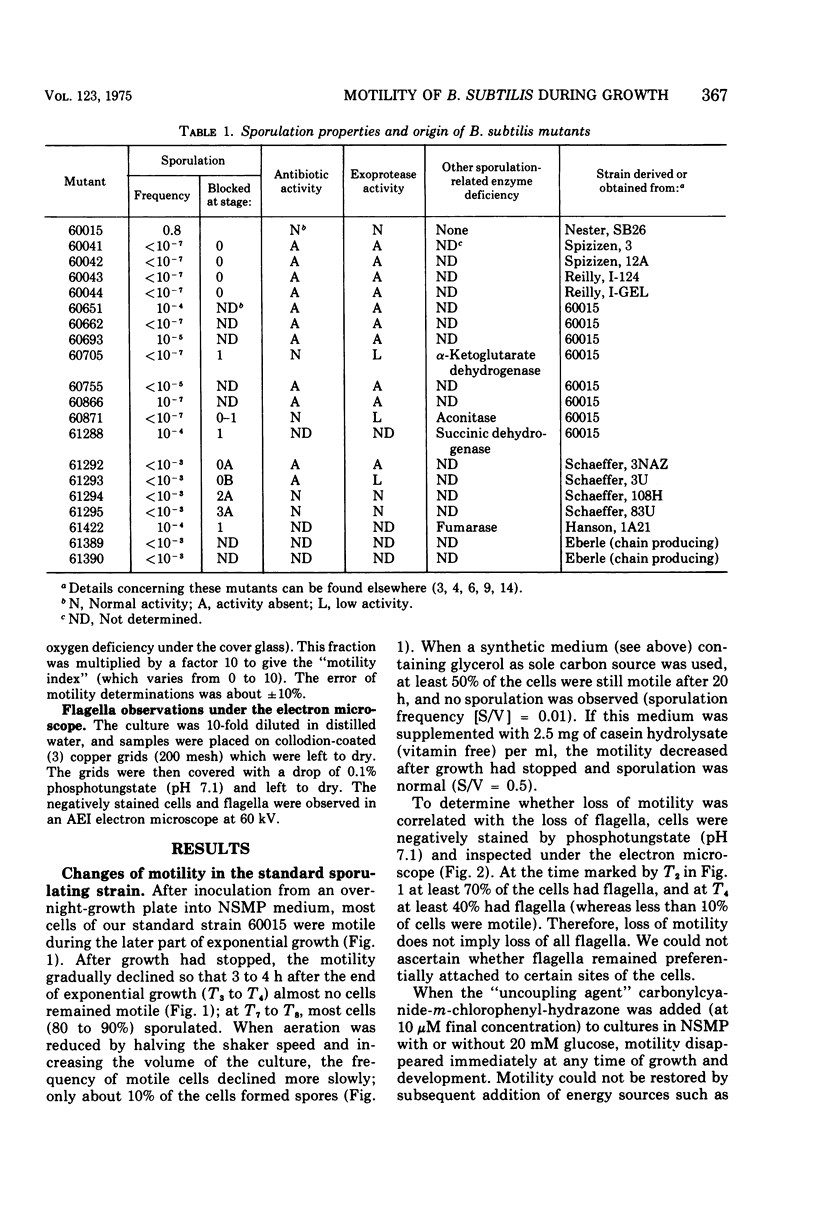
Abstract
The change of motility and the presence of flagella were followed throughout growth and sporulation in a standard sporulating strain and in 19 cacogenic sporulation mutants of Bacillus subtilis. For the standard strain, the fraction of motile cells decreased during the developmental period to less than 10% at T4. Motility was lost well before the cells lose their flagella. Conditions reducing the decrease of motility also reduced sporulation: motile cells never contained spores. The decrease of motility was not coupled with a decrease in the cellular concentration of adenosine 5'-triphosphate or a decline in oxygen consumption, but an uncoupling agent immediately destroyed motility at any time. Apparently, motility decreased during development because it became increasingly uncoupled from the energy generating systems of the cell. The motility of sporulation mutants decreased after the end of growth at the same time as or earlier than the motility of the standard strain; the early decrease of motility in an aconitase mutant, but not that in an alpha-ketoglurate dehydrogenase mutant, could be avoided by addition of L-glutamate. Sporulation or related events such as extracellular antibiotic or protease production were not needed for the motility decline.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Freese E. Sporulation of bacilli, a model of cellular differentiation. Curr Top Dev Biol. 1972;7:85–124. doi: 10.1016/s0070-2153(08)60070-8. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Mildner G., Spizizen J. Early blocked asporogenous mutants of Bacillus subtilis 168. I. Isolation and characterization of mutants resistant to antibiotic(s) produced by sporulating Bacillus subtilis 168. Mol Gen Genet. 1971;112(2):104–109. doi: 10.1007/BF00267488. [DOI] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]




