Abstract
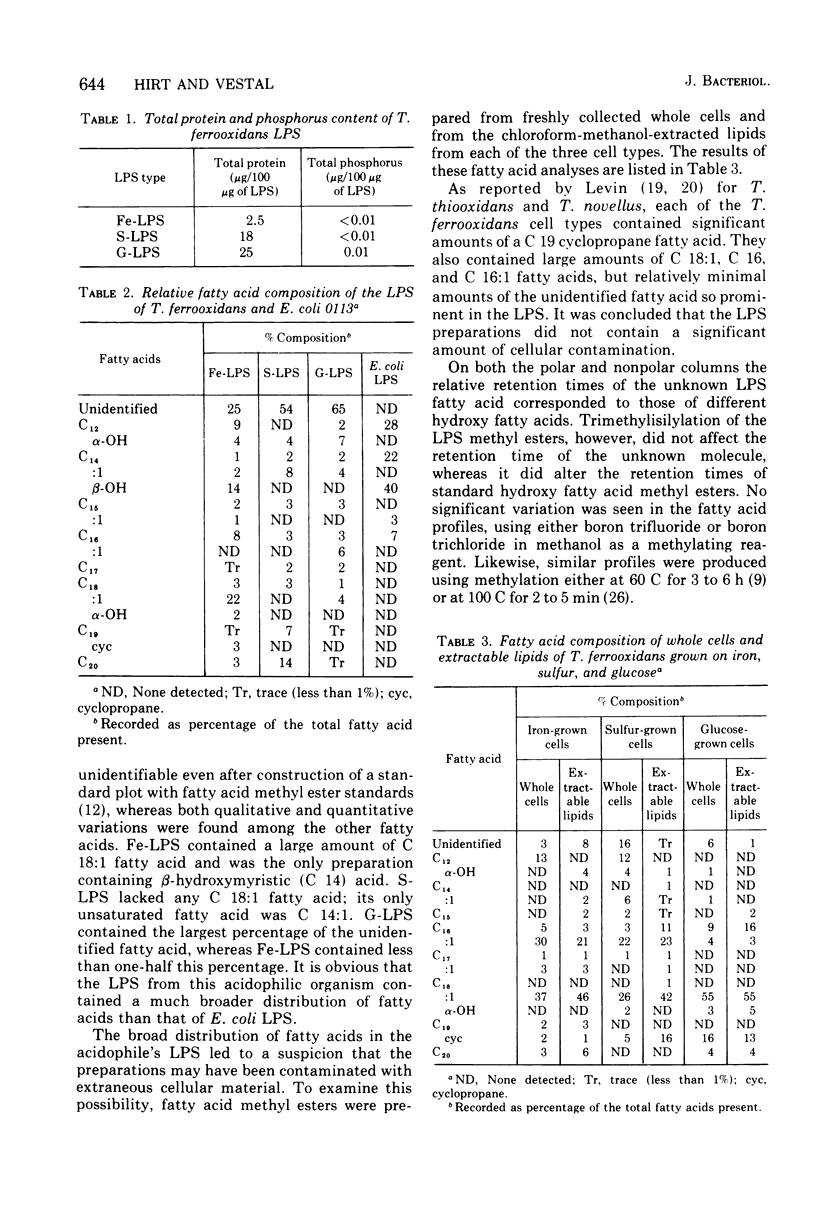
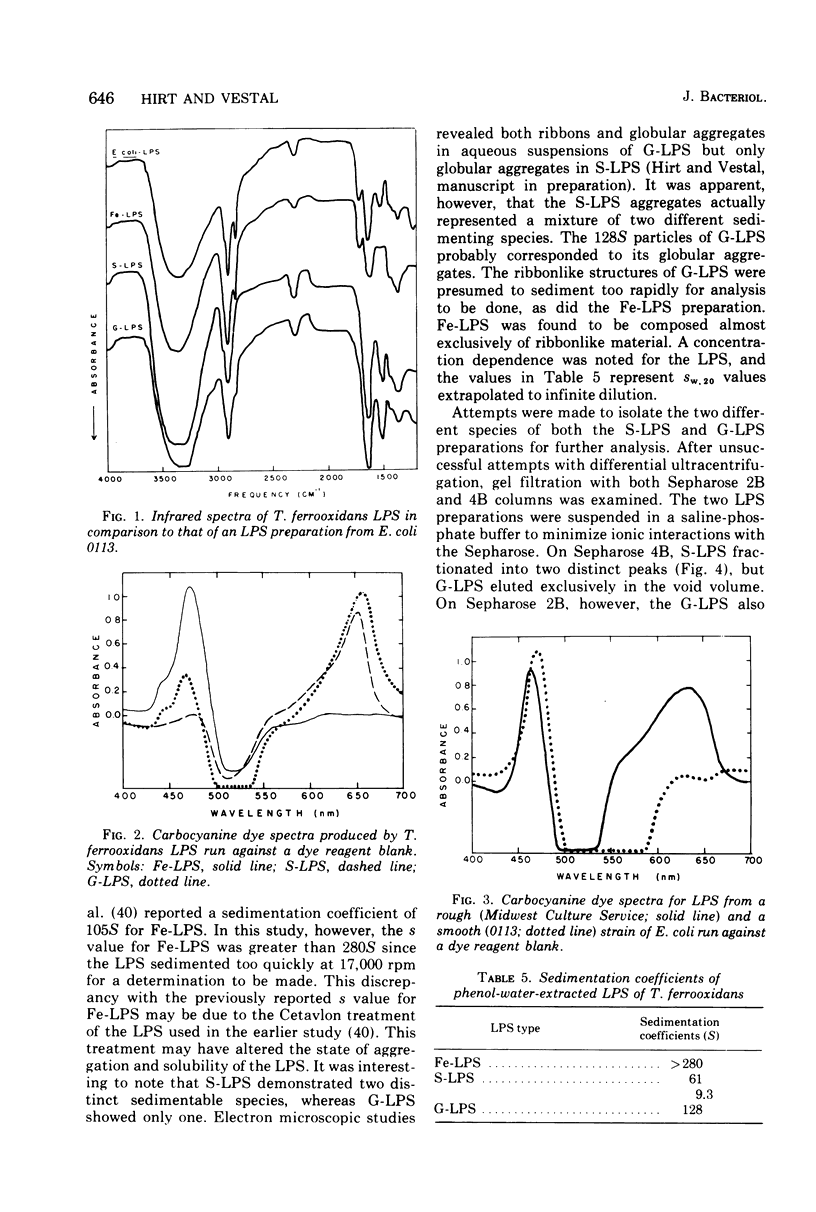
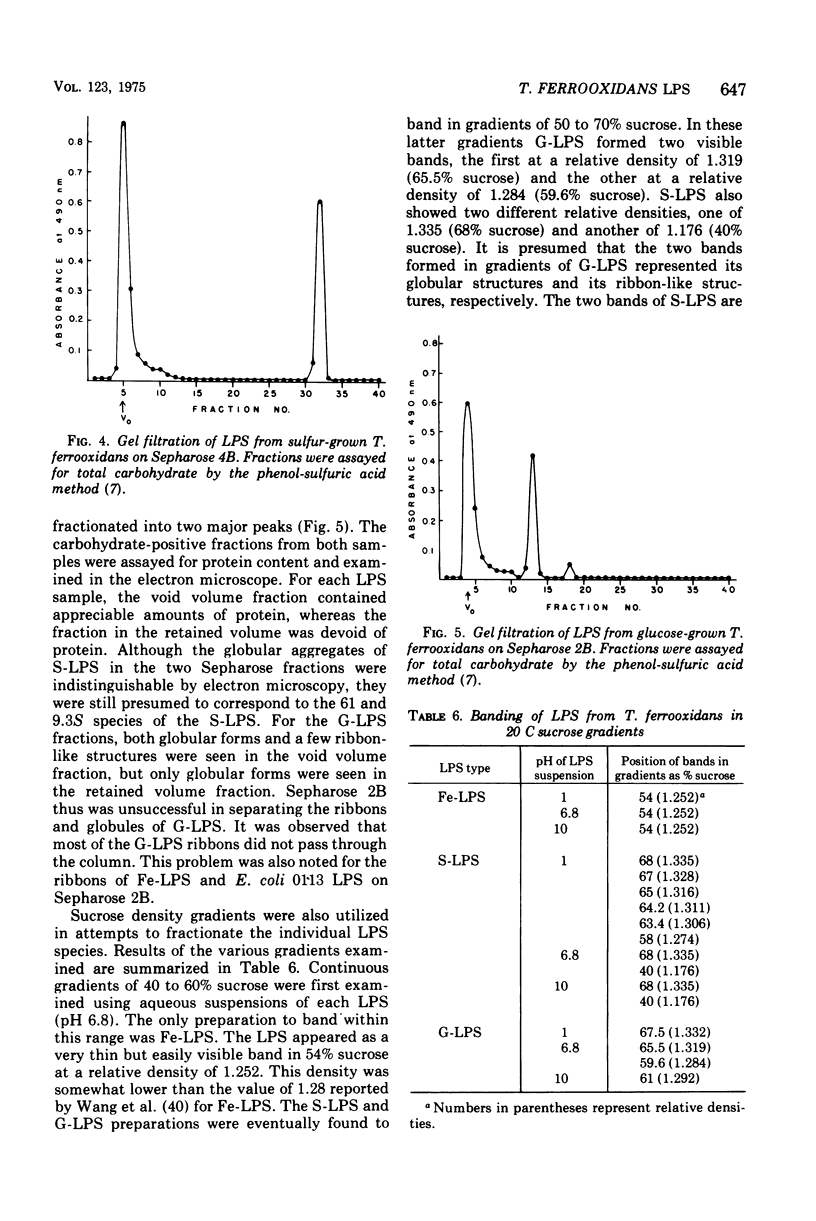
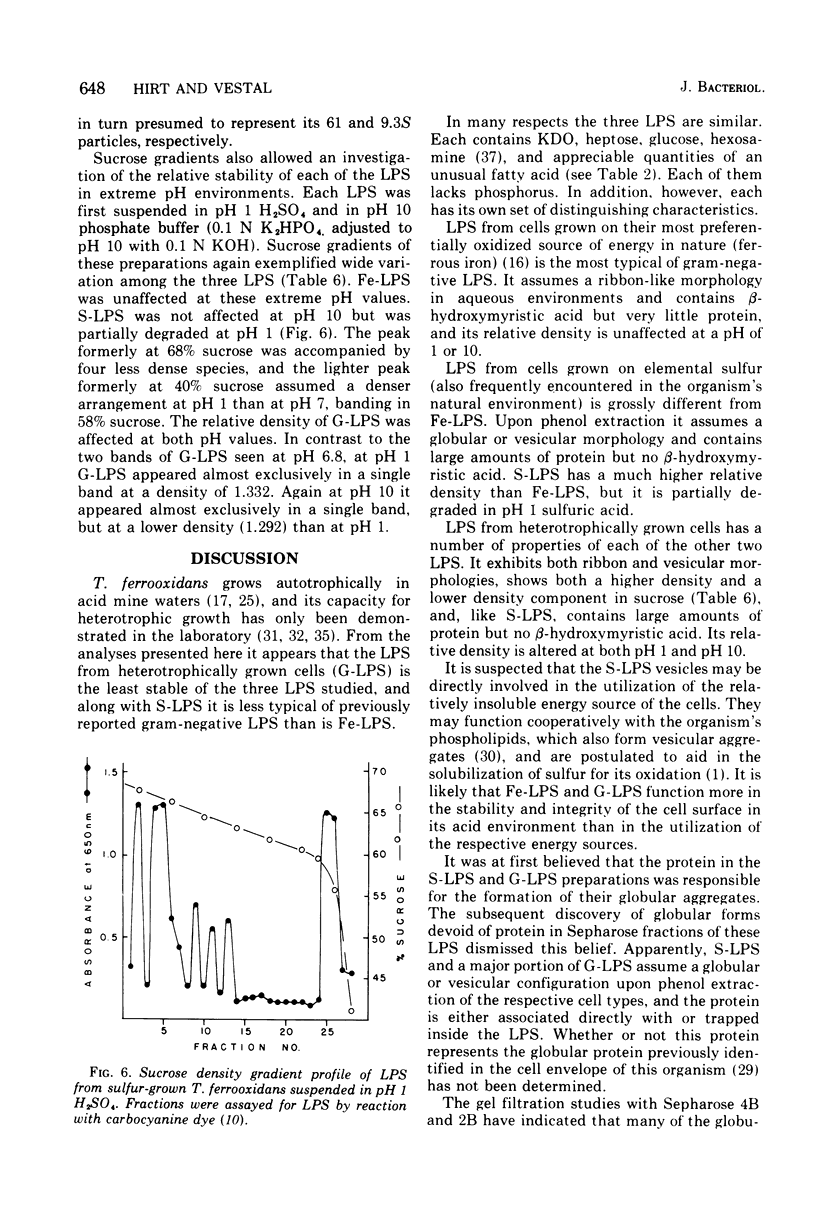
The lipopolysaccharides (LPS) of the obligate acidophile Thiobacillus ferroxidans grown on iron, sulfur, and glucose as energy sources were examined for various physical and chemical properties. Both qualitative and quantitative variation were found among the three preparations. The LPS extracted from iron-grown cells (Fe-LPS) contained less than 3% protein compared to 18 to 25% in LPS extracted from either sulfur-grown cells (S-LPS) or glucose-grown cells (G-LPS). S-LPS showed two distinct sedimentable species, 61S and 9.3S, which could be fractionated on a column of Sepharose 4B. The relative densities of both S-LPS and G-LPS were found to be significantly greater than that of Fe-LPS. Spectral differences were noted when each LPS was reacted with a carbocyanine dye. Fe-LPS showed a single absorbance maximum at 472 nm, S-LPS displayed its maximum at 650 nm, and G-LPS showed two maxima, the first at 468 nm and the other at 655 nm. Analysis of the methyl ester derivatives of the LPS fatty aicds using gas chromatography-mass spectrometry revealed the presence of a very stable species, tentatively identified as a methoxy methyl ester with a formula of CH3-3-C10H10-COOCH3, as the major component from each LPS. beta-Hydroxymyristic acid was found only in Fe-LPS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agate A. D., Korczynski M. S., Lundgren D. G. Extracellular complex from the culture filtrate of Ferrobacillus ferrooxidans. Can J Microbiol. 1969 Mar;15(3):259–264. doi: 10.1139/m69-048. [DOI] [PubMed] [Google Scholar]
- Agate A. D., Vishniac W. Iron transport by phospholipids in a two phase system containing water and n-pentanol. Chem Phys Lipids. 1972 Nov;9(3):247–254. doi: 10.1016/0009-3084(72)90005-9. [DOI] [PubMed] [Google Scholar]
- BLAYLOCK B. A., NASON A. ELECTRON TRANSPORT SYSTEMS OF THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. I. CYTOCHROME C-CONTAINING IRON OXIDASE. J Biol Chem. 1963 Oct;238:3453–3462. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BRALEY S. A., Sr, KINSEL N. A., LEATHEN W. W. Ferrobacillus ferrooxidans: a chemosynthetic autotrophic Bacterium. J Bacteriol. 1956 Nov;72(5):700–704. doi: 10.1128/jb.72.5.700-704.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo C., Lundgren D. G. Iron oxidation by cell envelopes of Thiobacillus ferrooxidans. Can J Microbiol. 1974 Dec;20(12):1647–1652. doi: 10.1139/m74-256. [DOI] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom R. D. A colorimetric method for the determination of mucopolysaccharides and other acidic polymers. Anal Biochem. 1969 Jun;29(3):421–432. doi: 10.1016/0003-2697(69)90327-3. [DOI] [PubMed] [Google Scholar]
- JAMES A. T. Qualitative and quantitative determination of the fatty acids by gas-liquid chromatography. Methods Biochem Anal. 1960;8:1–59. doi: 10.1002/9780470110249.ch1. [DOI] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Korczynski M. S., Agate A. D., Lundgren D. G. Phospholipids from the chemoautotroph Ferrobacillus ferrooxidans. Biochem Biophys Res Commun. 1967 Nov 30;29(4):457–462. doi: 10.1016/0006-291x(67)90505-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landesman J., Duncan D. W., Walden C. C. Oxidation of inorganic sulfur compounds by washed cell suspensions of Thiobacillus ferrooxidans. Can J Microbiol. 1966 Oct;12(5):957–964. doi: 10.1139/m66-129. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Levin R. A. Effect of cultural conditions on the fatty acid composition of Thiobacillus novellus. J Bacteriol. 1972 Nov;112(2):903–909. doi: 10.1128/jb.112.2.903-909.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. A. Fatty acids of Thiobacillus thiooxidans. J Bacteriol. 1971 Dec;108(3):992–995. doi: 10.1128/jb.108.3.992-995.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A. Relationship of structure and biological activity of bacterial endotoxins. Naturwissenschaften. 1971 Aug;58(8):397–409. doi: 10.1007/BF00591520. [DOI] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Brinson K. R., Heinzman M. W., Brady J. M. Transition of chemolithotroph Ferrobacillus ferrooxidans to obligate organotrophy and metabolic capabilities of glucose-grown cells. J Bacteriol. 1972 Jul;111(1):56–65. doi: 10.1128/jb.111.1.56-65.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Wilkinson R. F., Jr Growth of Ferrobacillus ferrooxidans on organic matter. J Bacteriol. 1969 Jan;97(1):256–260. doi: 10.1128/jb.97.1.256-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Aleem M. I. Phospholipid metabolism in Ferrobacillus ferrooxidans. J Bacteriol. 1969 Jul;99(1):142–150. doi: 10.1128/jb.99.1.142-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita R., Lundgren D. G. Utilization of glucose and the effect of organic compounds on the chemolithotroph Thiobacillus ferrooxidans. J Bacteriol. 1971 Oct;108(1):328–333. doi: 10.1128/jb.108.1.328-333.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J. C., Wang C. S., Alaupovic P. Degradative effect of phenol on endotoxin and lipopolysaccharide preparations from Serratia marcescens. J Bacteriol. 1974 Feb;117(2):786–795. doi: 10.1128/jb.117.2.786-795.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G., Milner K. C. Toxic and immunological differences among lipopolysaccharides from Thiobacillus ferrooxidans grown autotrophically and heterotrophically. Can J Microbiol. 1973 Nov;19(11):1335–1339. doi: 10.1139/m73-215. [DOI] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- Wang W. S., Korczynski M. S., Lundgren D. G. Cell envelope of an iron-oxidizing bacterium: studies of lipopolysaccharide and peptidoglycan. J Bacteriol. 1970 Oct;104(1):556–565. doi: 10.1128/jb.104.1.556-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. S., Lundgren D. G. Peptidoglycan of a chemolithotrophic bacterium, Ferrobacillus ferrooxidans. J Bacteriol. 1968 May;95(5):1851–1856. doi: 10.1128/jb.95.5.1851-1856.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Katz A., Drews G., Mayer H., Fromme I. Lipopolysaccharide containing L-acofriose in the filamentous blue-green alga Anabaena variabilis. J Bacteriol. 1974 Nov;120(2):672–678. doi: 10.1128/jb.120.2.672-678.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise G., Drews G., Jann B., Jann K. Identification and analysis of a lipopolysaccharide in cell walls of the blue-green alga Anacystis nidulans. Arch Mikrobiol. 1970;71(1):89–98. doi: 10.1007/BF00412238. [DOI] [PubMed] [Google Scholar]


