Abstract
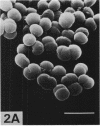
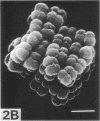
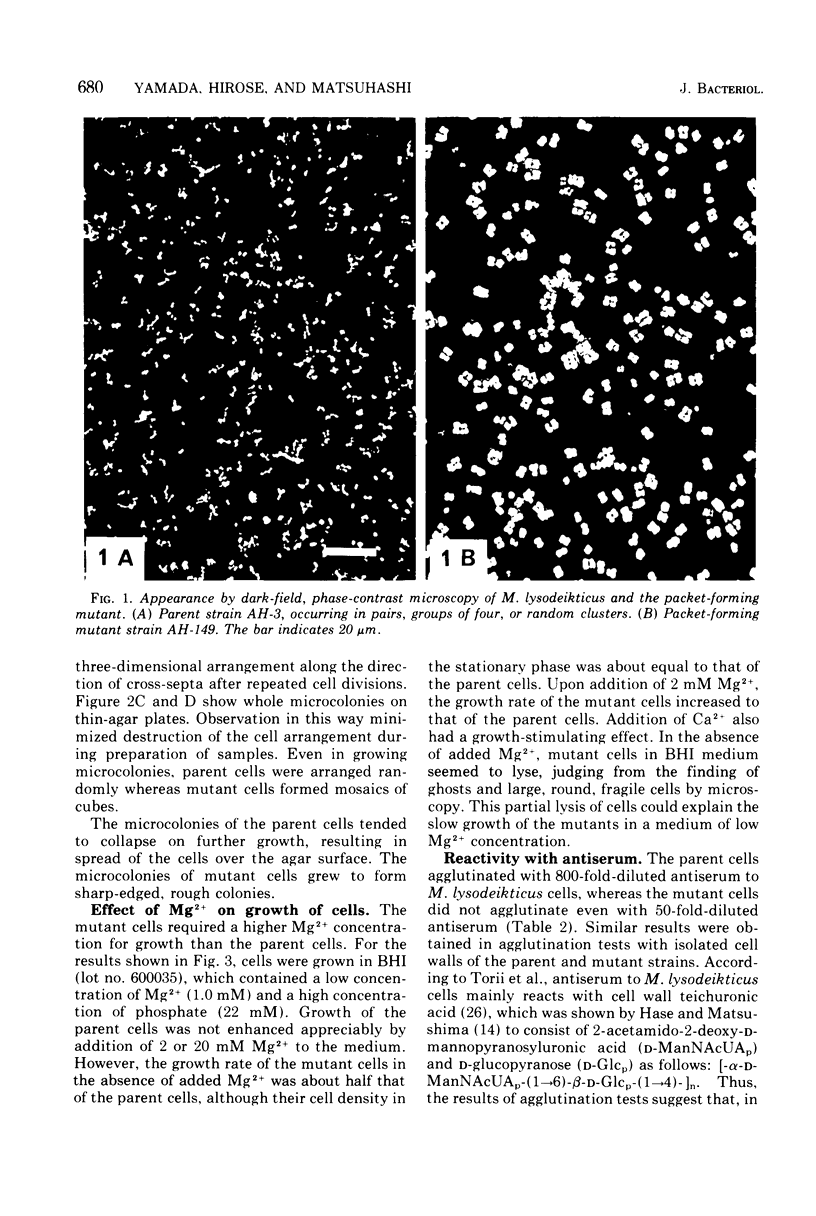
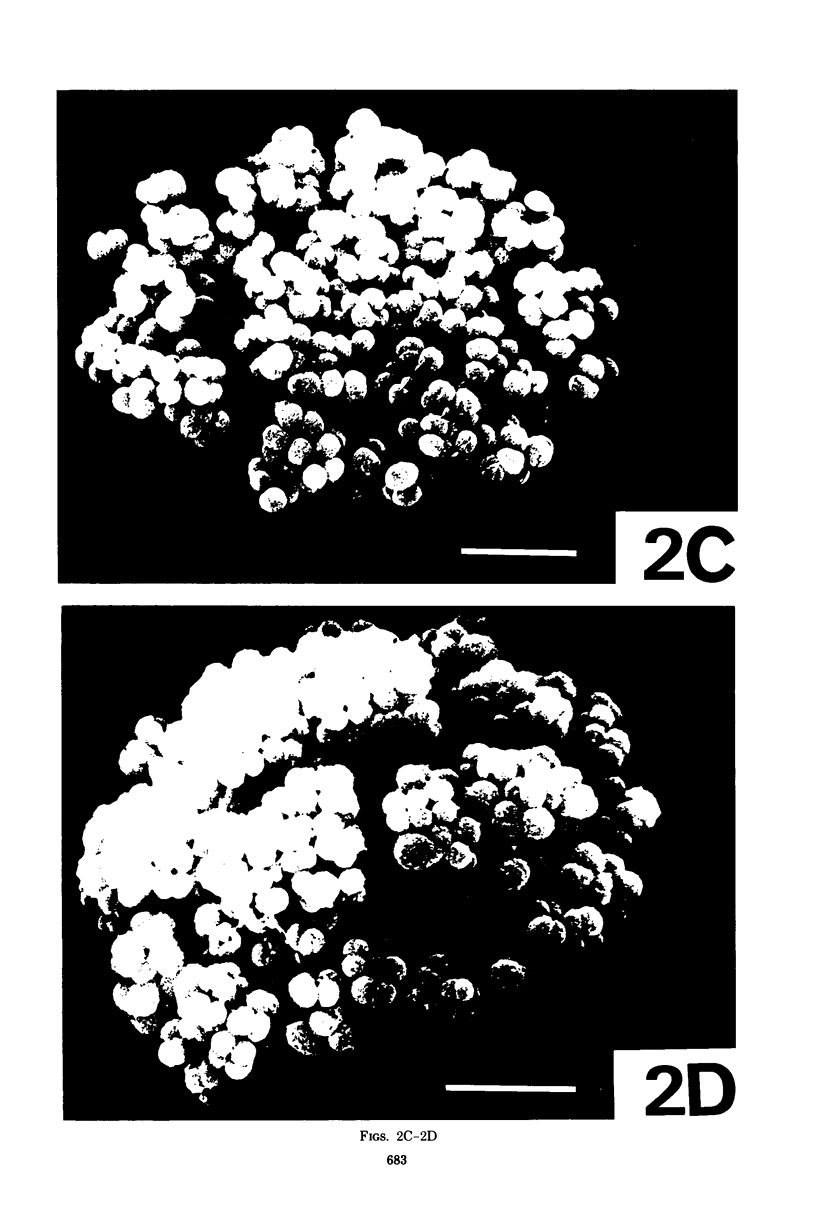
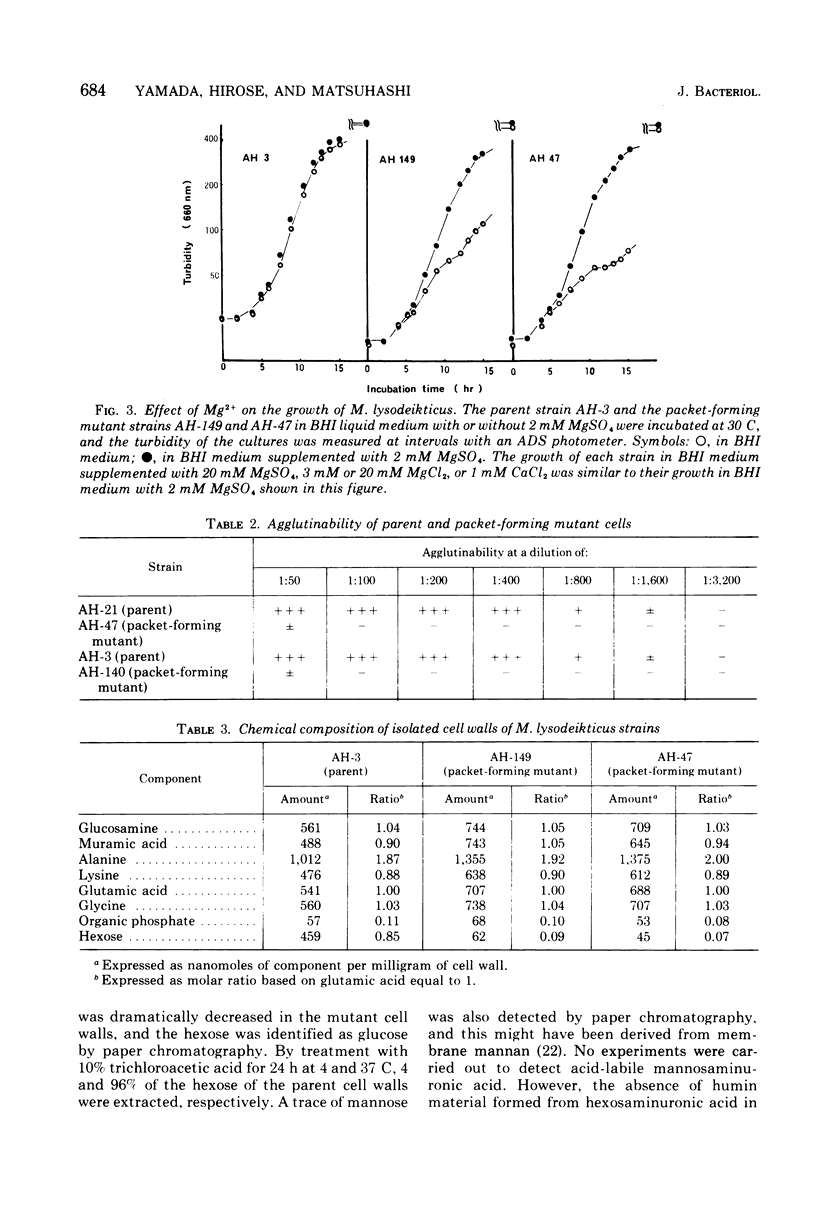
Morphological mutants of Micrococcus lysodeikticus (luteus) were isolated by treatment with N-methyl-N'-nitro-N-nitrosoguanidine. They occurred on plates in large, regular cell packets, whereas the parent cells usually grew as groups of two or four cells or as short chains. The mutants required a much higher concentration of Mg2+ for growth than the parent cells. The concentrations of Mg2+ and other components of the culture medium tested did not significantly affect the morphology of either the parent or mutant strains. The mutant strains were not agglutinated by antiserum to M. lysodeikticus, which mainly interacts with teichuronic acid on the cell surface, and chemical analysis of isolated cell walls of the mutants indicated the absence of teichuronic aicd. No significant differences were detected between the parent and mutant strains in the amounts of other cell wall components, e.g., peptidoglycan, protein, and teichoic acid. They possible roles of teichuronic acid in cell separation and attachment of divalent cations are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J., Blumsom N. L. The teichoic acids. Adv Enzymol Relat Areas Mol Biol. 1968;30:223–253. doi: 10.1002/9780470122754.ch5. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- CANALE-PAROLA E., BORASKY R., WOLFE R. S. Studies on Sarcina ventriculi. III. Localization of cellulose. J Bacteriol. 1961 Feb;81:311–318. doi: 10.1128/jb.81.2.311-318.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- CZERKAWSKI J. W., PERKINS H. R., ROGERS H. J. A study of the composition and structure of the cell-wall mucopeptide of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:468–474. doi: 10.1042/bj0860468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Mutant of Bacillus subtilis demonstrating the requirement of lysis for growth. J Bacteriol. 1971 Feb;105(2):629–636. doi: 10.1128/jb.105.2.629-636.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schultes L. M. Transformation in Micrococcus lysodeikticus. J Gen Microbiol. 1969 Feb;55(2):307–317. doi: 10.1099/00221287-55-2-307. [DOI] [PubMed] [Google Scholar]
- Kloos W. E. Transformation of Micrococcus lysodeikticus by various members of the family micrococcaceae. J Gen Microbiol. 1969 Dec;59(2):247–255. doi: 10.1099/00221287-59-2-247. [DOI] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosypal S., Rosypalová A., Horejs J. The classification of micrococci and staphylococci based on their DNA base composition and adansonian analysis. J Gen Microbiol. 1966 Aug;44(2):281–292. doi: 10.1099/00221287-44-2-281. [DOI] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper J. W., Winter C. G. Role of cell wall antolysin in chain formation by a mutant strain of Streptococcus faecalis. Biochim Biophys Acta. 1973 Feb 28;297(2):333–342. doi: 10.1016/0304-4165(73)90080-9. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M., Sakakibara K., Iida S., Kobayashi K. Immunochemical studies on a polysaccharide from Micrococcus lysodeikticus containing glucose and N-acetyl-mannosaminuronic acid. Biken J. 1973 Mar;16(1):11–16. [PubMed] [Google Scholar]
- WOLIN H. L., NAYLOR H. B. Basic nutritional requirements of Micrococcus lysodeikticus. J Bacteriol. 1957 Aug;74(2):163–167. doi: 10.1128/jb.74.2.163-167.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]