Abstract
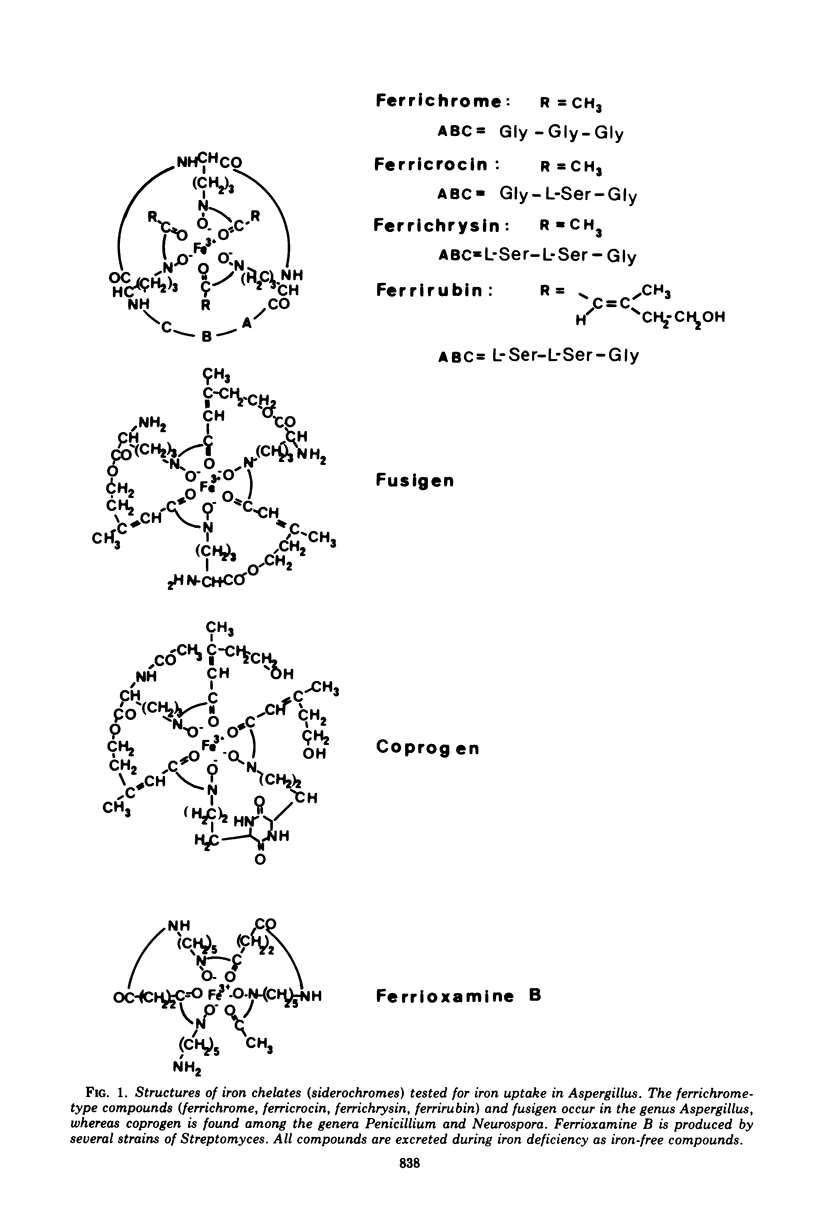
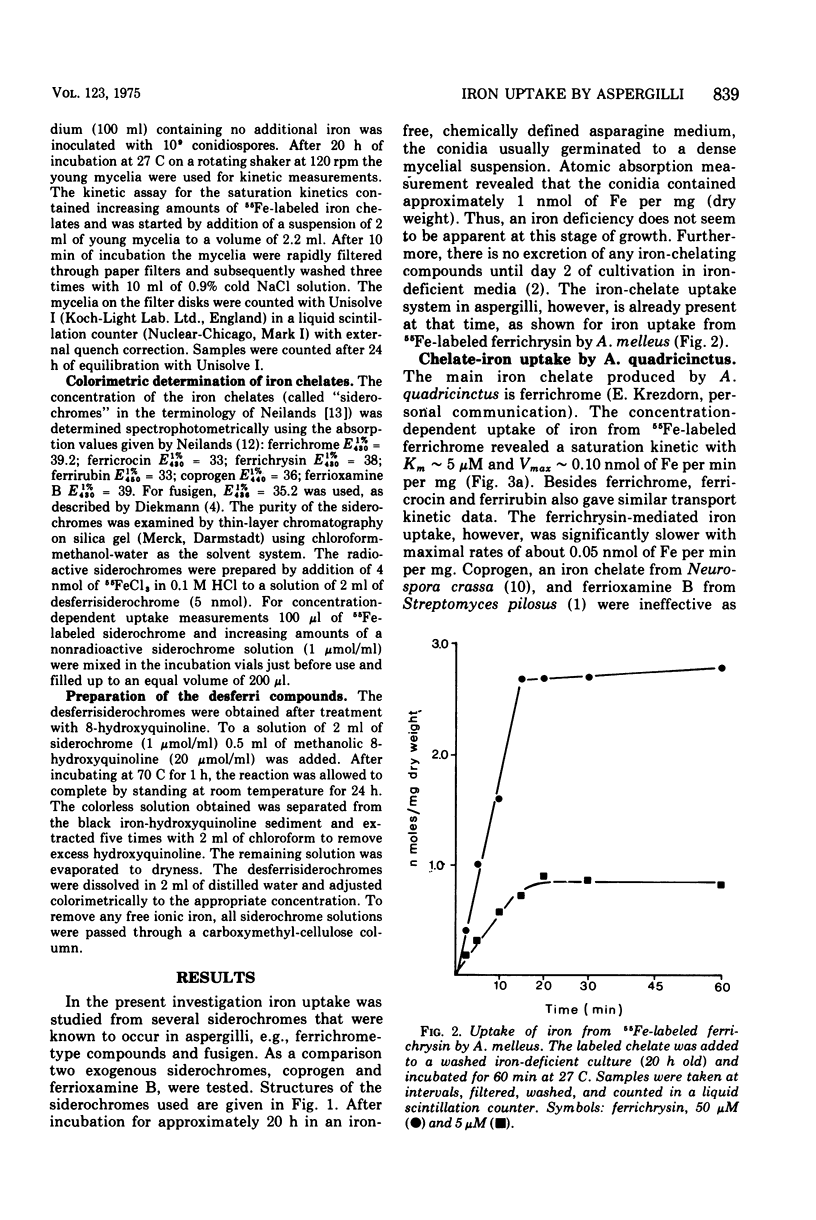
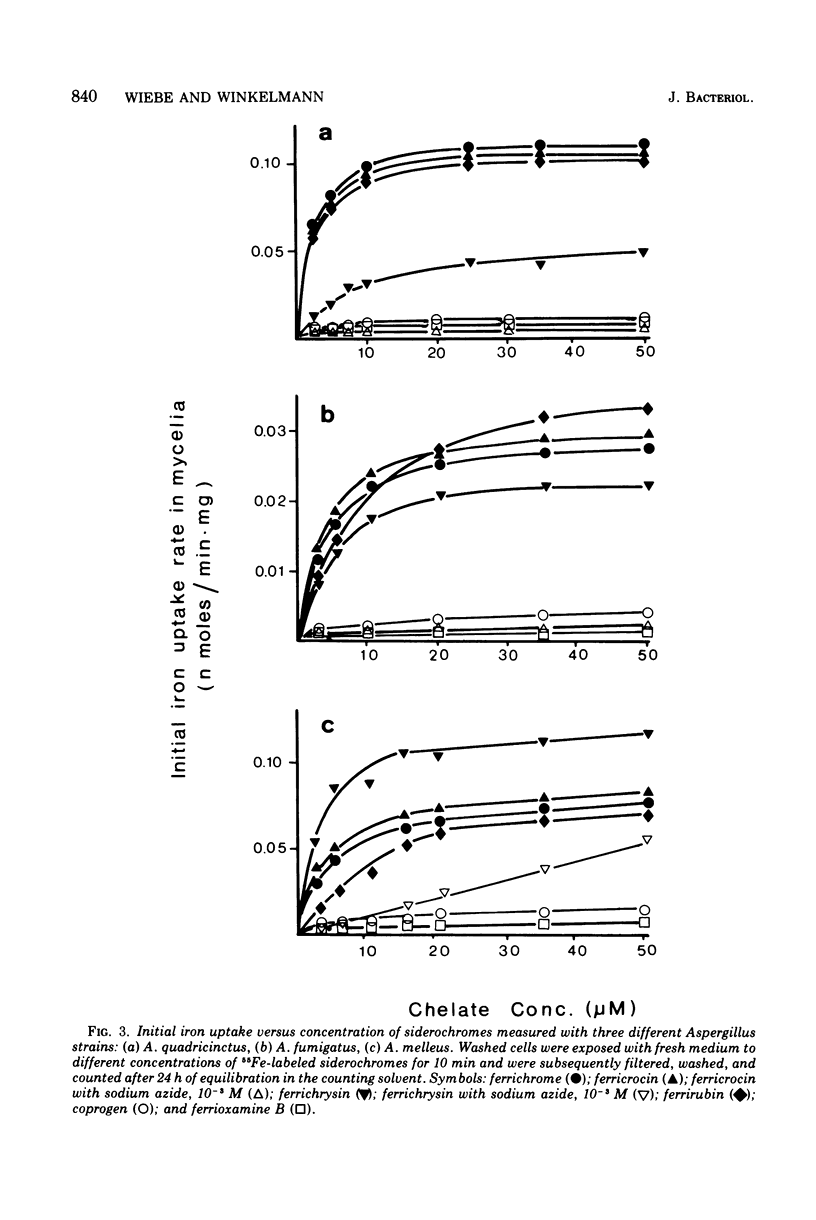
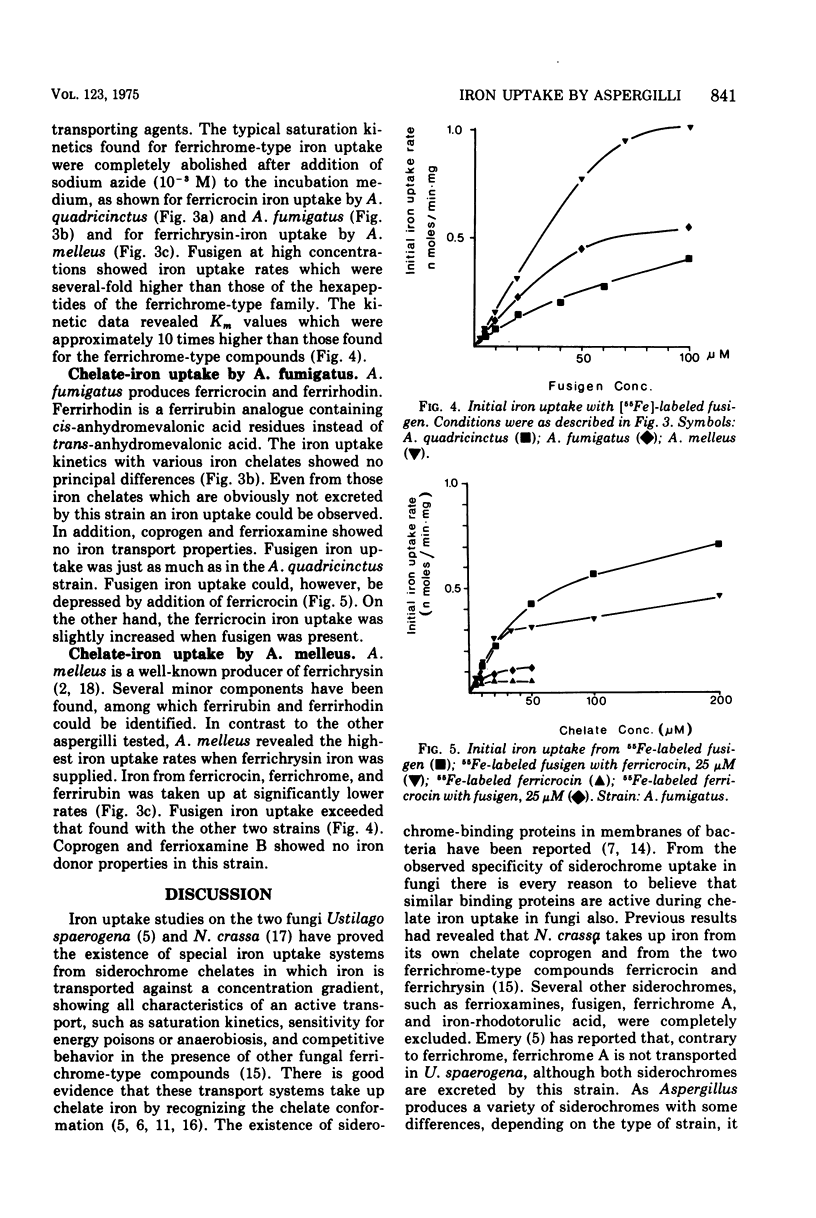
Three strains of the fungus Aspergillus, Aspergillus quadricinctus (E. Yuill), A. fumigatus (Fresenius), and A. melleus (Yukawa), each producing different iron-chelating compounds during iron-deficient cultivation, were used for 55Fe3+ uptake measurements. Iron from chelates of the ferrichrome-type family was taken up by young mycelia of all strains tested, irrespective of the ferrichrome-type compound these strains predominantly produce in low-iron cultures. Ferrichrysin-producing strains, however, seem to favor ferrichrysin iron uptake, whereas ferrichrome, ferricrocin, and even ferrirubin showed similar iron transport properties in all of these strains. Compared to iron uptake from ferrichrome-type compounds (Km approximately 4 uM) iron uptake from fusigen revealed completely different kinetic values (Km approximately 50 to 80 muM). Iron from exogenous chelates, e.g., from coprogen produced by Neurospora crassa for ferrioxamine B produced by Streptomyces pilosus, can obviously not be taken up by Aspergillus, confirming the pronounced specificity of chelate-iron transport in fungi.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crueger W., Zähner H. Stoffwechselprodukte von Mikroorganismen. 70. Uber den Einfluss der Kohlenstoffquelle auf die Sideraminbildung von Aspergillus melleus Yukawa. Arch Mikrobiol. 1968;63(4):376–384. [PubMed] [Google Scholar]
- Diekmann H. Stoffwechselprodukte von Mikroorganismen. 56. Fusigen--ein neues Sideramin aus Pilzen. Arch Mikrobiol. 1967;58(1):1–5. [PubMed] [Google Scholar]
- Emery T. Role of ferrichrome as a ferric ionophore in Ustilago sphaerogena. Biochemistry. 1971 Apr 13;10(8):1483–1488. doi: 10.1021/bi00784a033. [DOI] [PubMed] [Google Scholar]
- Ernst J., Winkelmann G. Metabolic products of microorganisms. 135. Uptake of iron by Neurospora crassa. IV. Iron transport properties of semisynthetic coprogen derivatives. Arch Microbiol. 1974;100(3):271–282. doi: 10.1007/BF00446323. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Llinás M., Klein M. P., Neilands J. B. The solution conformation of the ferrichromes. V. The hydrogen exchange kinetics of ferrichrome analogues; the conformational state of the peptides. J Biol Chem. 1973 Feb 10;248(3):924–931. [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G., Barnekow A., Ilgner D., Zähner H. Stoffwechselprodukte von Mikroorganismen. 120. Eisenaufnahme bei Neurospora crassa. II. Regulation der Sideraminbildung und Inhibition des Eisentransportes durch Metallanaloge des Coprogens. Arch Mikrobiol. 1973;92(4):285–300. [PubMed] [Google Scholar]
- Winkelmann G. Metabolic products of microorganisms. 132. Uptake of iron by Neurospora crassa. 3. Iron transport studies with ferrichrome-type compounds. Arch Mikrobiol. 1974 Jun 7;98(1):39–50. [PubMed] [Google Scholar]
- Winkelmann G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 115. Eisenaufnahme bei Neurospora crassa. I. Zur Spezifität des Eisentransportes. Arch Mikrobiol. 1973;88(1):49–60. [PubMed] [Google Scholar]


