Abstract
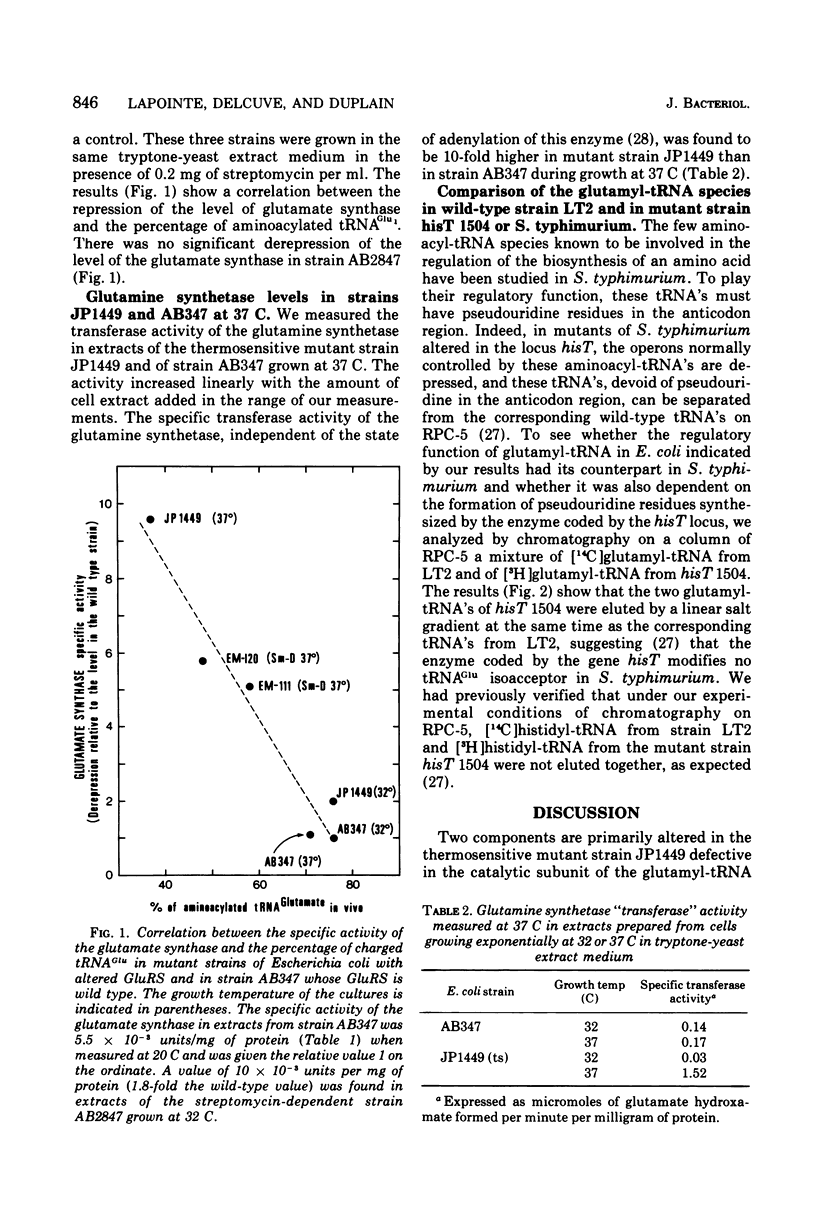
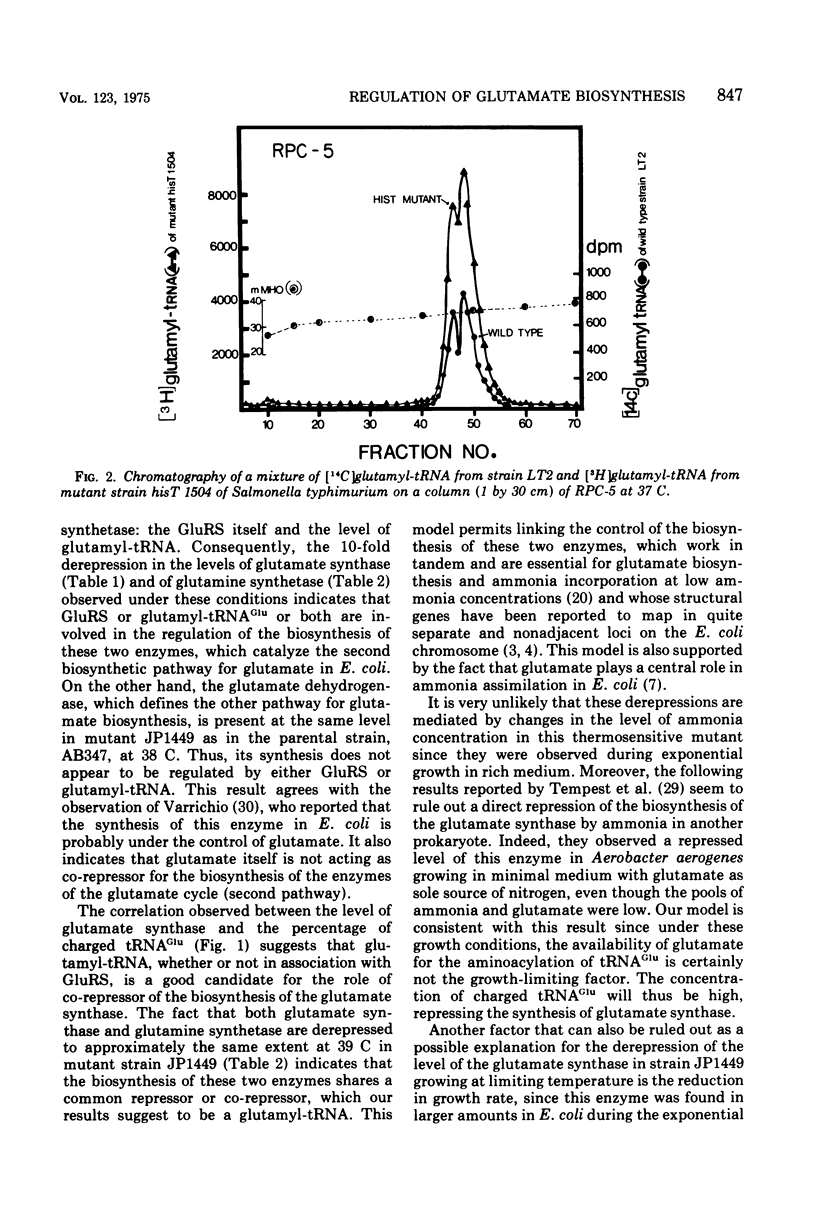
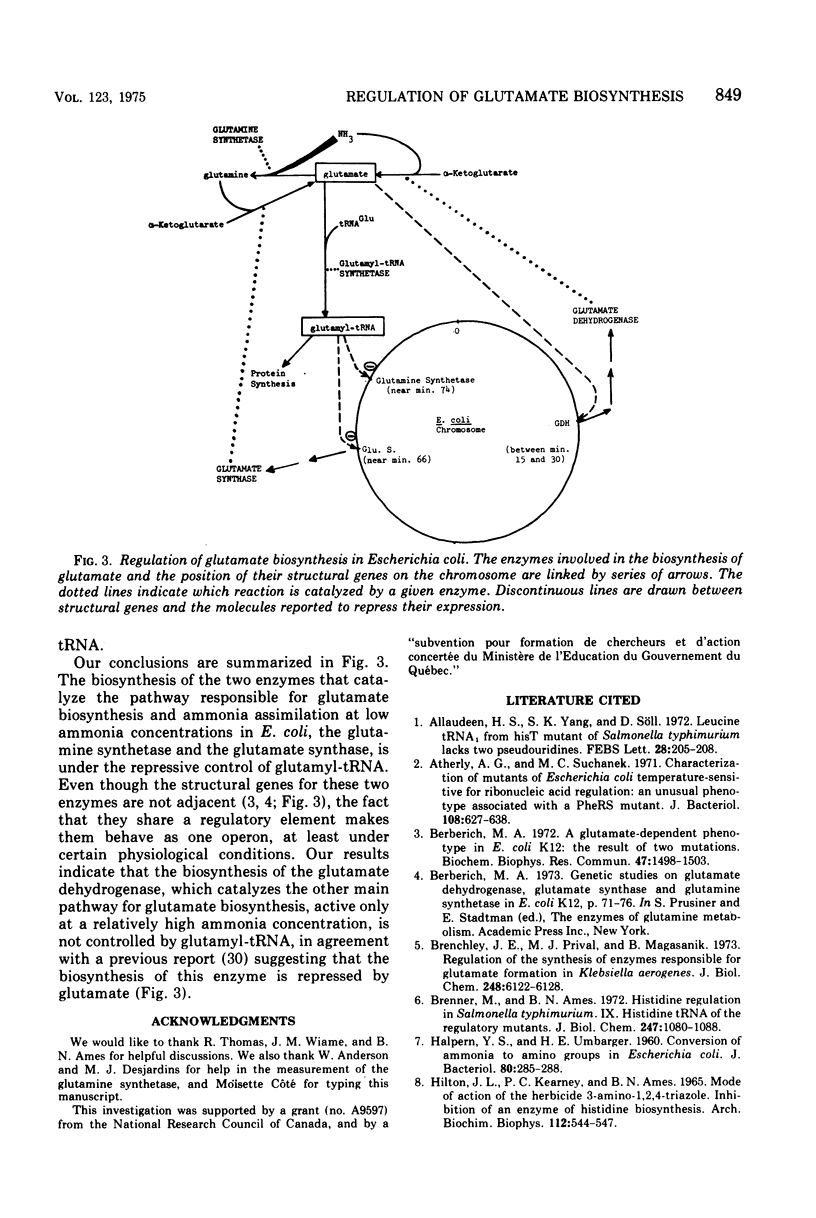
The levels of glutamate synthase and of glutamine synthetase are both derepressed 10-fold in strain JP1449 of Escherichia coli carrying a thermosensitive mutation in the glutamyl-transfer ribonucleic acid (tRNA) synthetase and growing exponentially but at a reduced rate at a partially restrictive temperature, compared with the levels in strain AB347 isogenic with strain JP1449 except for this thermosensitive mutation and the marker aro. These two enzymes catalyze one of the two pathways for glutamate biosynthesis in E. coli, the other being defined by the glutamate dehydrogenase. We observed a correlation between the percentage of charged tRNAGlu and the level of glutamate synthase in various mutants reported to have an altered glutamyl-tRNA synthetase activity. These results suggest that a glutamyl-tRNA might be involved in the repression of the biosynthesis of the glutamate synthase and of the glutamine synthetase and would couple the regulation of the biosynthesis of these two enzymes, which can work in tandem to synthesize glutamate when the ammonia concentration is low in E. coli but whose structural genes are quite distant from each other. No derepression of the level of the glutamate dehydrogenase was observed in mutant strain JP1449 under the conditions where the levels of the glutamine synthetase and of the glutamate synthase were derepressed. This result indicates that the two pathways for glutamate biosynthesis in E. coli are under different regulatory controls. The glutamate has been reported to be probably the key regulatory element of the biosynthesis of the glutamate dehydrogenase. Our results indicate that the cell has chosen the level of glutamyl-tRNA as a more sensitive probe to regulate the biosynthesis of the enzymes of the other pathway, which must be energized at a low ammonia concentration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Yang S. K., Söll D. Leucine tRNA(1) from HisT mutant of Salmonella typhimurium lacks two pseudouridines. FEBS Lett. 1972 Dec 1;28(2):205–208. doi: 10.1016/0014-5793(72)80713-0. [DOI] [PubMed] [Google Scholar]
- Atherly A. G., Suchanek M. C. Characterization of mutants of Escherichia coli temperature-sensitive for ribonucleic acid regulation: an unusual phenotype associated with a phenylalanyl transfer ribonucleic acid synthetase mutant. J Bacteriol. 1971 Nov;108(2):627–638. doi: 10.1128/jb.108.2.627-638.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich M. A. A glutamate-dependent phenotype in E. coli K12: the result of two mutations. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1498–1503. doi: 10.1016/0006-291x(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E., Prival M. J., Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973 Sep 10;248(17):6122–6128. [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- Holmquist R., Jukes T. H., Pangburn S. Evolution of transfer RNA. J Mol Biol. 1973 Jun 25;78(1):91–116. doi: 10.1016/0022-2836(73)90430-0. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Atherly A. G., Barrett A. Synthesis of stable RNA in stringent Escherichia coli cells in the absence of charged transfer RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):689–692. doi: 10.1073/pnas.70.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Irie T., Yoshida M., Takeishi K., Ukita T. The primary structure of yeast glutamic acid tRNA specific to the GAA codon. Biochim Biophys Acta. 1974 Oct 11;366(2):168–181. doi: 10.1016/0005-2787(74)90331-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Delcuve G. Thermosensitive mutants of Escherichia coli K-12 altered in the catalytic Subunit and in a Regulatory factor of the glutamy-transfer ribonucleic acid synthetase. J Bacteriol. 1975 May;122(2):352–358. doi: 10.1128/jb.122.2.352-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. 3. Influence of the 46K protein on the affinity of the 56K glutamyl transfer ribonucleic acid synthetase for its substrates. J Biol Chem. 1972 Aug 25;247(16):4982–4985. [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and properties. J Biol Chem. 1972 Aug 25;247(16):4966–4974. [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Prival M. J., Brenchley J. E., Tyler B. M., DeLeo A. B., Streicher S. L., Bender R. A., Paris C. G. Glutamine synthetase as a regulator of enzyme synthesis. Curr Top Cell Regul. 1974;8(0):119–138. doi: 10.1016/b978-0-12-152808-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Murgola E. J., Adelberg E. A. Mutants of Escherichia coli K-12 with an altered glutamyl-transfer ribonucleic acid synthetase. J Bacteriol. 1970 Jul;103(1):178–183. doi: 10.1128/jb.103.1.178-183.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi Z., Saneyoshi M., Harada F., Hara H., Nishimura S. Presumed anticodon structure of glutamic acid tRNA from E. coli: a possible location of a 2-thiouridine derivative in the first position of the anticodon. Biochem Biophys Res Commun. 1970 Aug 24;40(4):866–872. doi: 10.1016/0006-291x(70)90983-6. [DOI] [PubMed] [Google Scholar]
- Ohashi Ziro, Harada Fumio, Nishimura Susumu. Primary sequence of glutamic acid tRNA II from Escherichia coli. FEBS Lett. 1972 Feb 1;20(2):239–241. doi: 10.1016/0014-5793(72)80804-4. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Varricchio F. Control of glutamate dehydrogenase synthesis in Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):560–564. doi: 10.1016/0304-4165(69)90319-5. [DOI] [PubMed] [Google Scholar]


