Abstract
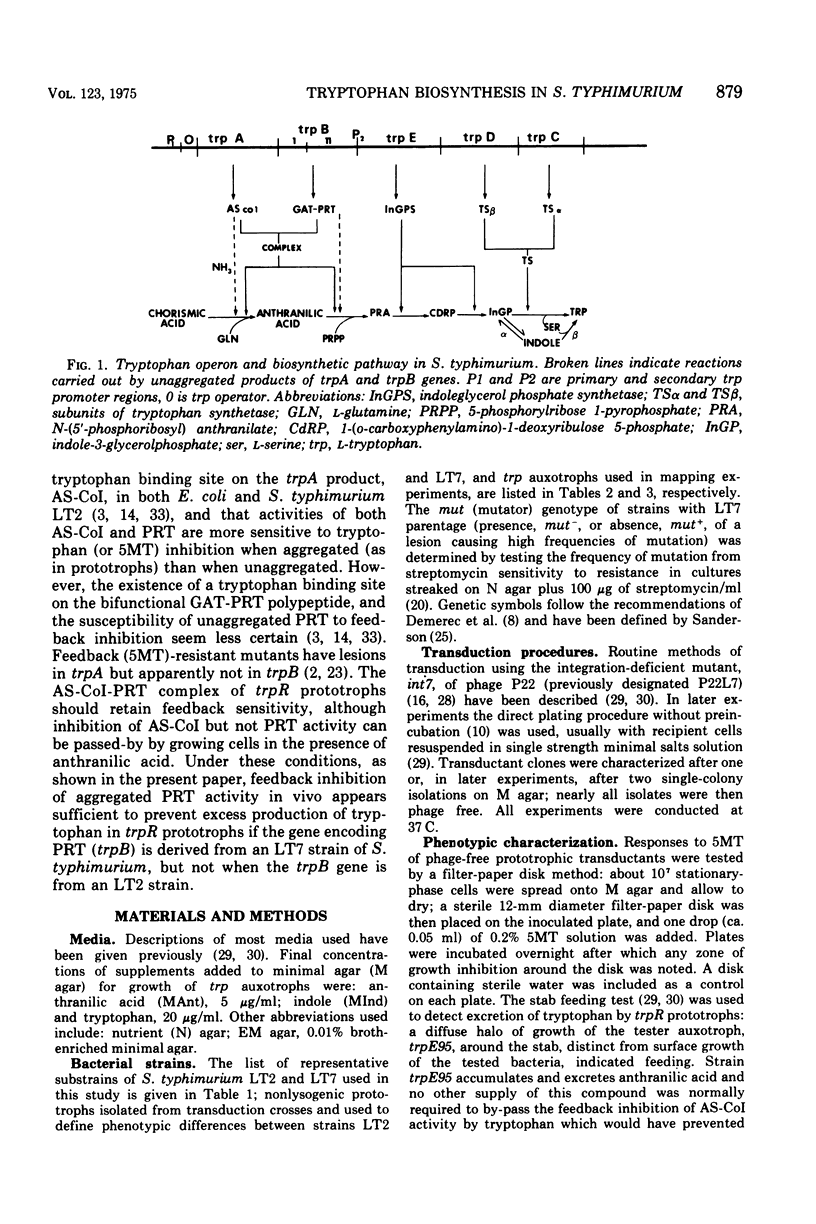
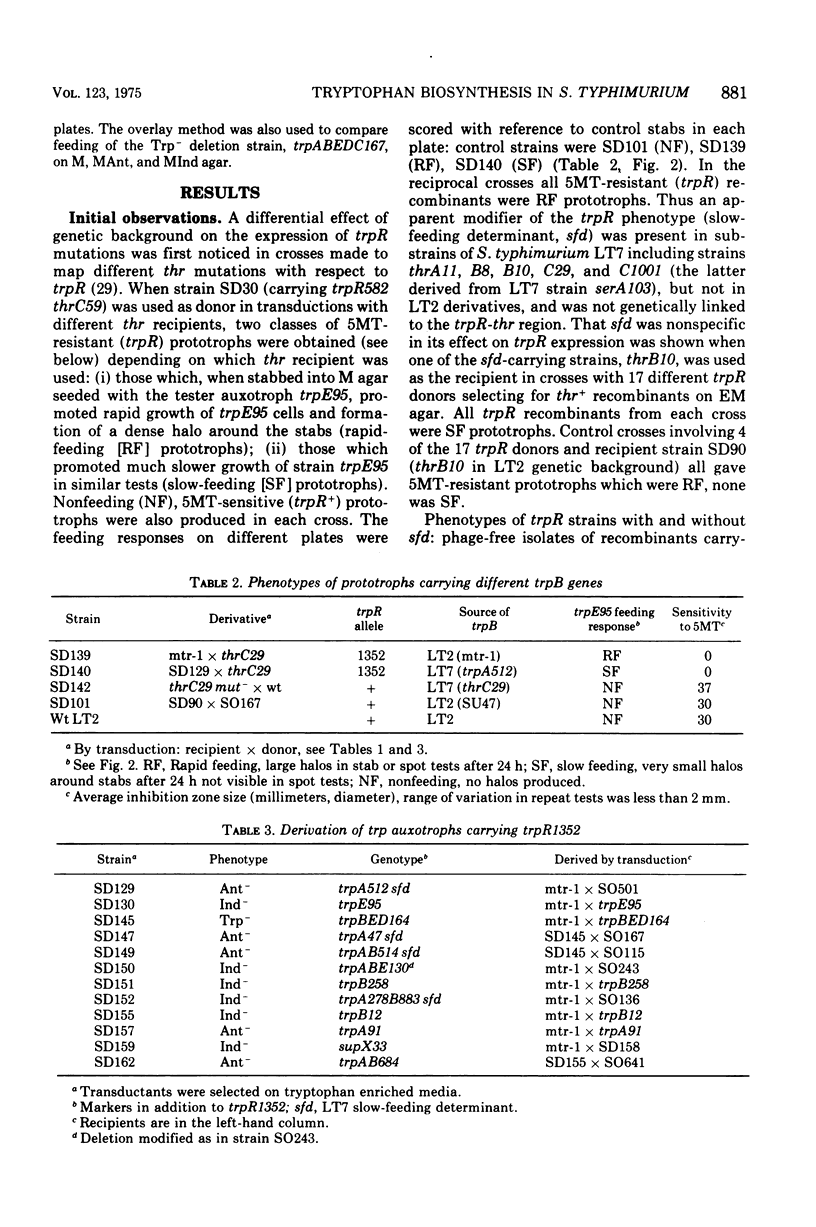
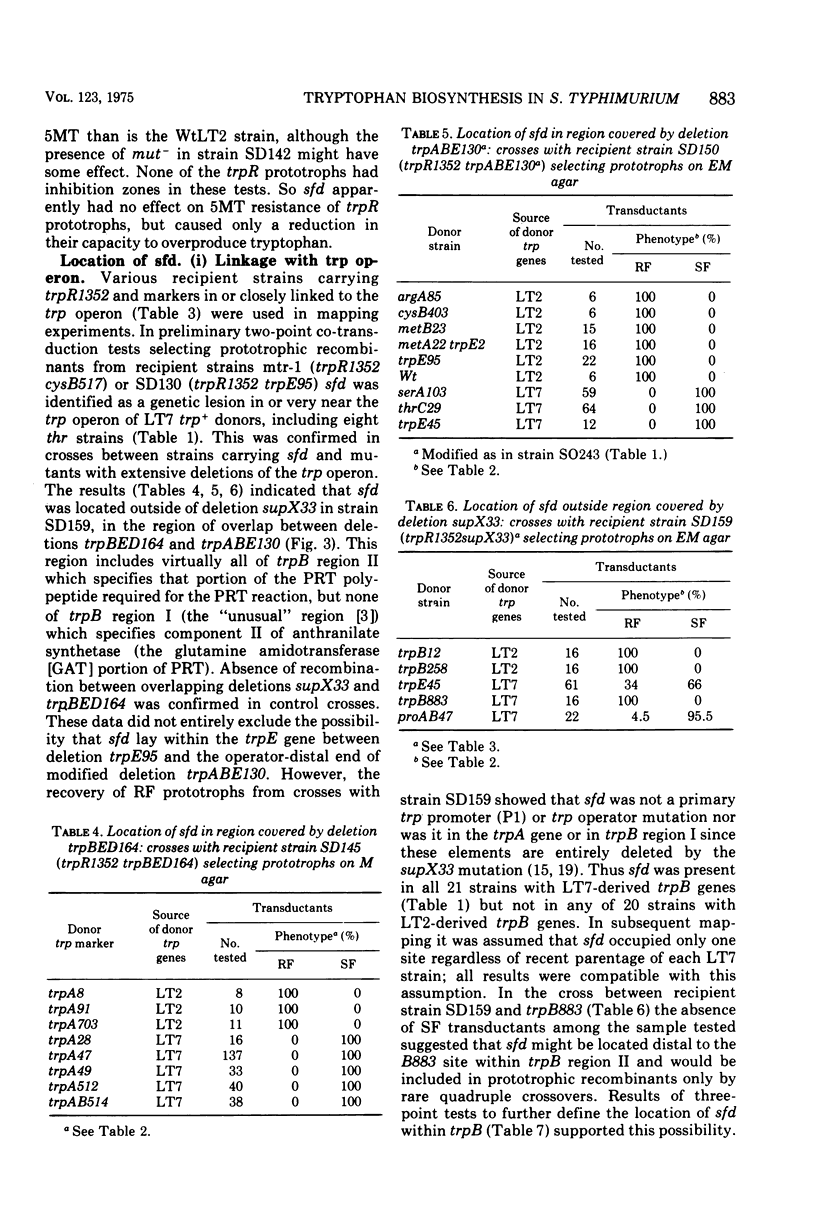
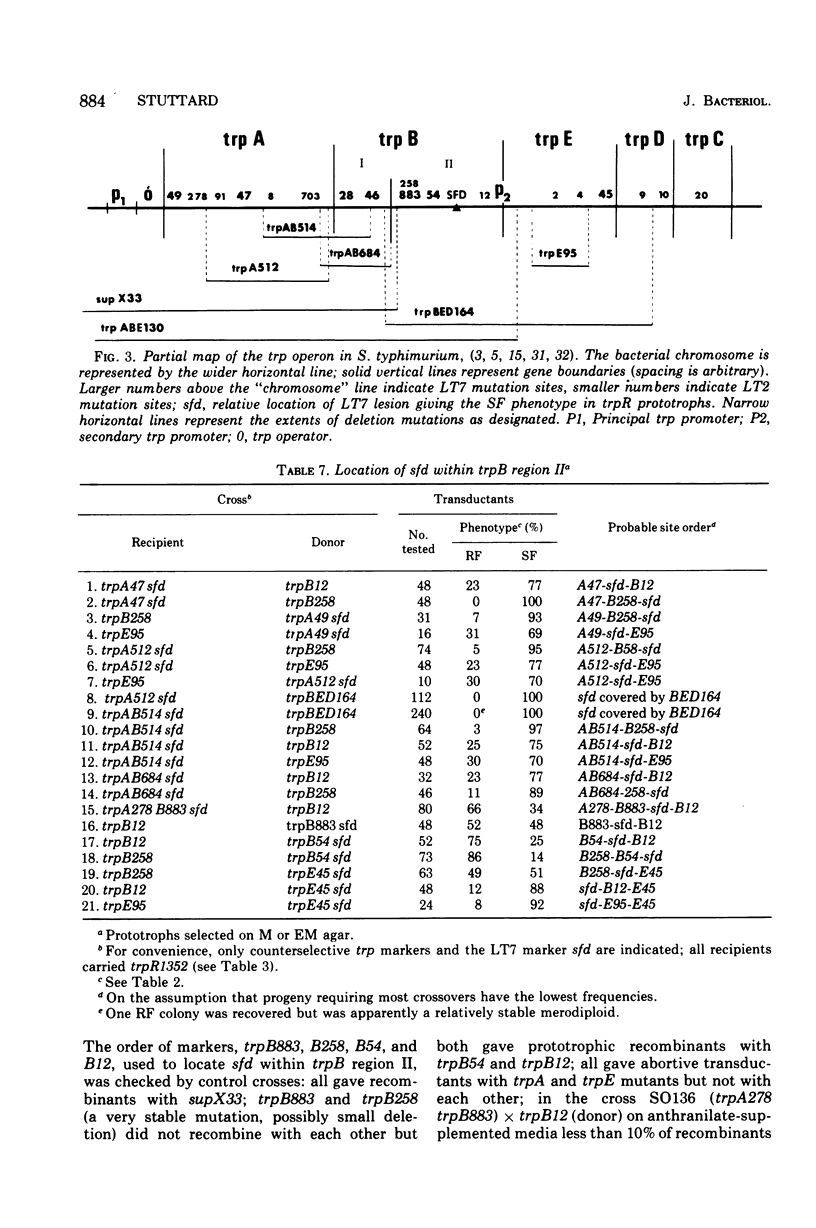
Salmonella typhimurium prototrophs carrying a trpR mutation synthesize tryptophan biosynthetic enzymes constitutively. When feedback inhibition of anthranilate synthetase but not 5'-phosphoribosylpyrophosphate phosphoribosyltransferase activity was by-passed by growing cells on media supplemented with anthranilic acid, all trpR prototrophs overproduced and excreted tryptophan. However, the rate of tryptophan production depended on both the ancestry of the trpR strain and the integrity of its trpA gene. Prototrophs with trp genes derived from S. typhimurium strain LT2 produced tryptophan more efficiently than those with trp genes derived from strain LT7. This strain difference was cryptic insofar as it did not affect the growth rate; it was revealed only as a rate-limiting step in the constitutive biosynthesis of tryptophan in the presence of anthranilic acid, and was due to a lesion in the LT7-derived trpB gene. Strains with LT7-derived trp genes bearing a deletion in trpA produced tryptophan as readily as LT2 trpR prototrophs. This indicated that LT7-specific 5-phosphoribosylpyrophosphate phosphoribosyltransferase must be aggregated with the trpA gene produce to give an observable reduction of constitutive tryptophan production. The discovery of this strain difference has particular implications for studies involving the activities of trpA and B genes and their products in S. typhimurium and may have general significance for other studies involving different strains of Salmonella.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balbinder E., Blume A. J., Weber A., Tamaki H. Polar and antipolar mutants in the tryptophan operon of Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2217–2229. doi: 10.1128/jb.95.6.2217-2229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinder E., Callahan R., 3rd, McCann P. P., Cordaro J. C., Weber A. R., Smith A. M., Angelosanto F. Regulatory mutants of the tryptophan operon of Salmonella typhimurium. Genetics. 1970 Sep;66(1):31–53. doi: 10.1093/genetics/66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boro H., Brenchley J. E. A new generalized transducing phage for Salmonella typhimurium LT2. Virology. 1971 Sep;45(3):835–836. doi: 10.1016/0042-6822(71)90208-x. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Colson C., Van Pel A. Host-controlled restriction mutants of Salmonella typhimurium. J Gen Microbiol. 1969 Sep;58(1):57–64. doi: 10.1099/00221287-58-1-57. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. J., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Catalytic and regulatory properties of aggregated and unaggregated forms of anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase. J Biol Chem. 1970 Mar 25;245(6):1424–1431. [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Balbinder E. Restoration of phosphoribosyl transferase activity by partially deleting the trpB gene in the tryptophan operon of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):877–885. doi: 10.1128/jb.112.2.877-885.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. Replication and lysogeny with phage P22 in Salmonella typhimurium. Curr Top Microbiol Immunol. 1972;58:135–156. doi: 10.1007/978-3-642-65357-5_4. [DOI] [PubMed] [Google Scholar]
- Li S. L., Hanlon J., Yanofsky C. Separation of anthranilate synthetase components I and II of Escherichia coli, Salmonella typhimurium, and Serratia marcescens and determination of their amino-terminal sequences by automatic Edman degradation. Biochemistry. 1974 Apr 9;13(8):1736–1744. doi: 10.1021/bi00705a028. [DOI] [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Miyake T, Demerec M. Proline Mutants of Salmonella Typhimurium. Genetics. 1960 Jun;45(6):755–762. doi: 10.1093/genetics/45.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T. Mutator Factor in Salmonella Typhimurium. Genetics. 1960 Jan;45(1):11–14. doi: 10.1093/genetics/45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Kuhn J. C., Somerville R. L. Feedback regulation in the anthranilate aggregate from wild type and mutant strains of Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):901–914. [PubMed] [Google Scholar]
- Pinel J. P., Chorover S. L. Inhibition by arousal of epilepsy induced by chlorambucil in rats. Nature. 1972 Mar 31;236(5344):232–234. doi: 10.1038/236232a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Squires C. L., Yanofsky C., Yang H. L., Zubay G. Regulation of in vitro transcription of the tryptophan operon by purified RNA polymerase in the presence of partially purified repressor and tryptophan. Nat New Biol. 1973 Oct 3;245(144):133–137. doi: 10.1038/newbio245133a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shimizu N., Hayashi M. In vitro repression of transcription of the tryptophan operon by trp repressor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1990–1994. doi: 10.1073/pnas.70.7.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Stuttard C., Dawson G. W. "Self-feeding" strain of Salmonella typhimurium with a mutation in the trpB gene and nutritional requirements of trpA gene mutants. J Bacteriol. 1969 Sep;99(3):779–783. doi: 10.1128/jb.99.3.779-783.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuttard C. Genetic analysis of thr mutations in Salmonella typhimurium. J Bacteriol. 1973 Oct;116(1):1–11. doi: 10.1128/jb.116.1.1-11.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuttard C. Location of trpR mutations in the serB-thr region of Salmonella typhimurium. J Bacteriol. 1972 Aug;111(2):368–374. doi: 10.1128/jb.111.2.368-374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuesthoff G., Bauerle R. H. Mutations creating internal promoter elements in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1970 Apr 14;49(1):171–196. doi: 10.1016/0022-2836(70)90384-0. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Henderson E. J. Tryptophan-mediated substrate inhibition of anthranilate-5-phosphoribosylpyrophosphate phosphoribosyltransferase. Biochem Biophys Res Commun. 1969 Apr 10;35(1):52–58. doi: 10.1016/0006-291x(69)90481-1. [DOI] [PubMed] [Google Scholar]