Abstract
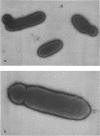
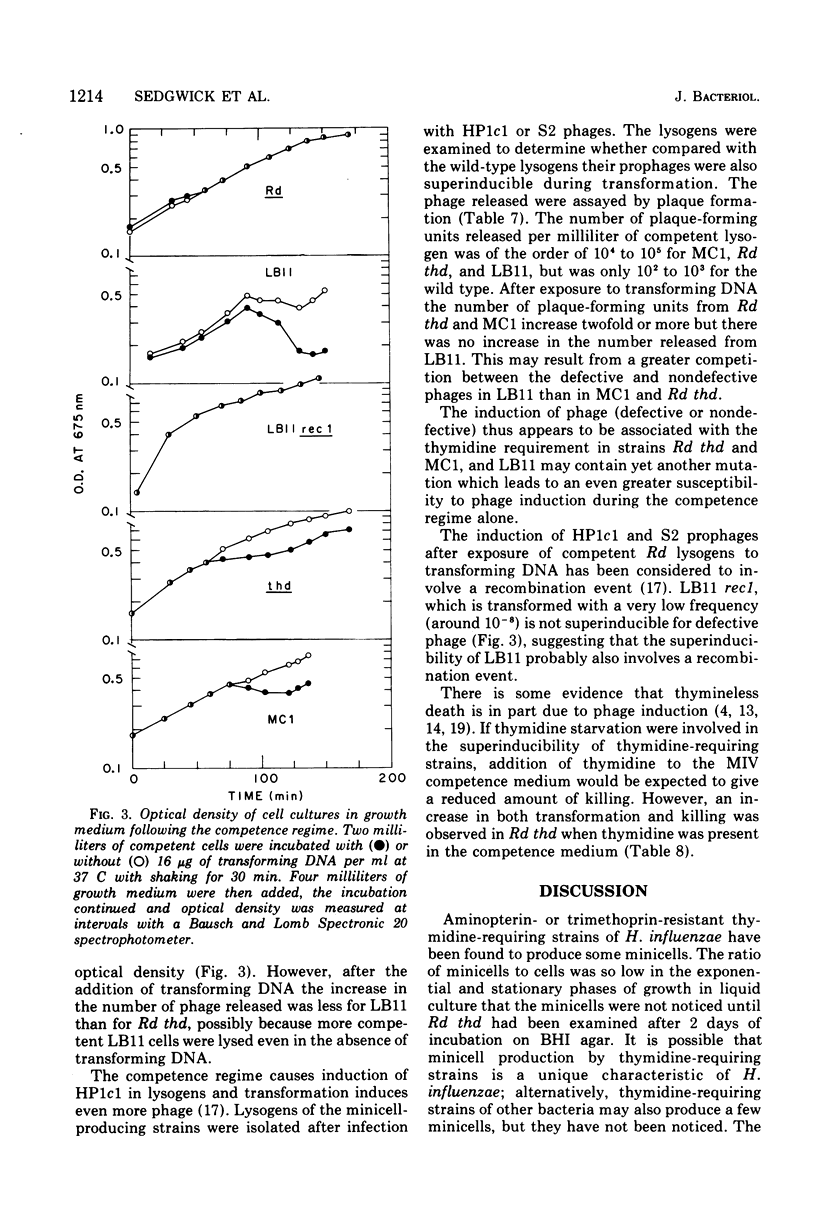
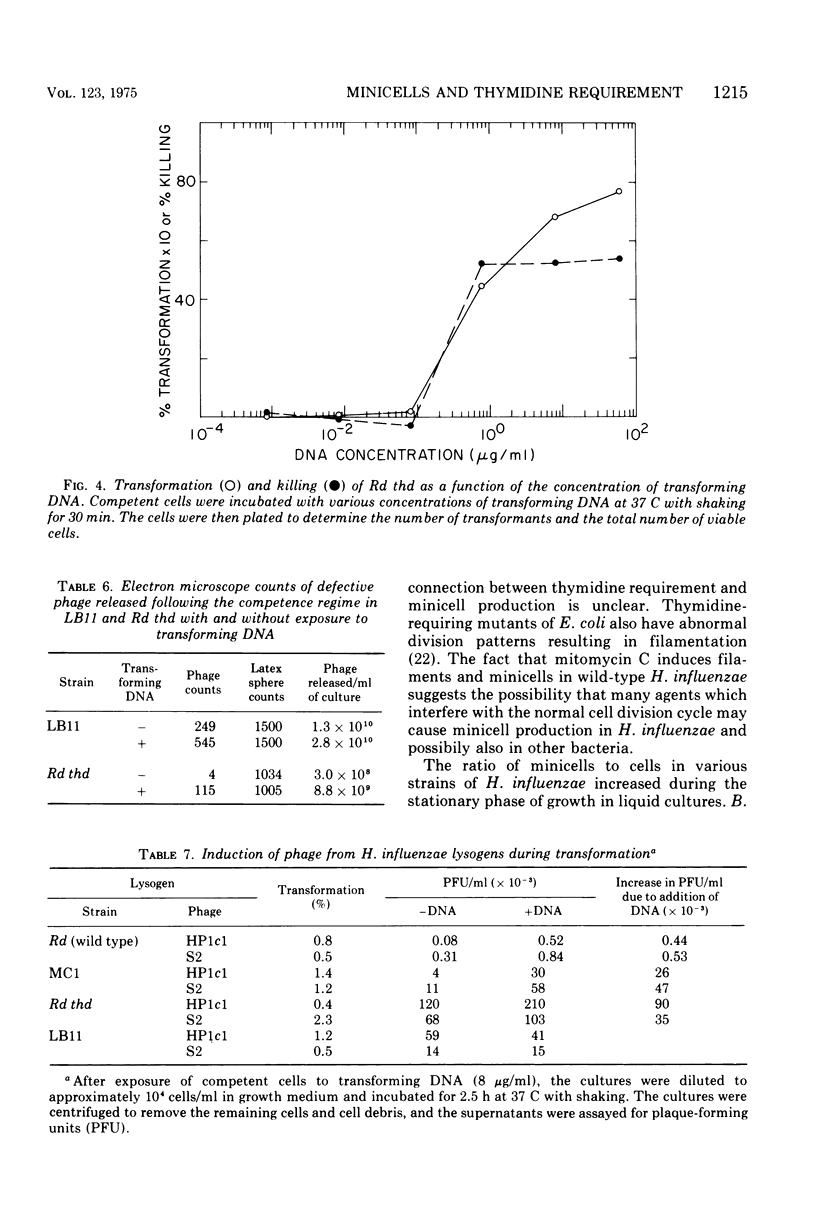
Aminopterin- or trimethoprin-resistant thymidine-requiring strains of Haemophilus influenzae produce minicells, and the ratio of minicells to cells increases during the stationary phase of growth. Strain LB11, isolated after mutagenesis of a thymidine-requiring strain (Rd thd), produces more minicells than the parent strain. The mutations involved in high frequency minicell production have been transferred into the wild type (strain Rd) by transformation. The thymidine requirement in the resulting strain, MCl, is essential for minicell production, since spontaneous revertants of MCl to prototrophy do not produce minicells. The ratio of minicells to cells was increased more than 10(3)-fold by differential centrifugation. The minicells contain little or no deoxyribonucleic acid (DNA). Phage HPlcl apparently cannot attach to minicells. Competent cells of LB11 and its thymidine-requiring parent strain produce defective phage as a result of exposure to transforming DNA, whereas only LB11 produces many defective phage in response to the competence regime alone. Competent HP1c1 and S2 lysogens of MC1 and Rd thd are also superinducible by transforming DNA, but competent LB11 lysogens produced about the same amount of HP1c1 or S2 phage with or without exposure to transforming DNA possibly because of competition between the induced defective phage and Hp1c1 or S2 phage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L. Breakage of parental DNA strands in Haemophilus influenzae by 313 nm radiation after replication in the presence of 5-bromodeoxyuridine. Biophys J. 1972 Nov;12(11):1573–1582. doi: 10.1016/S0006-3495(72)86183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Allison D. P., Setlow J. K. Bacteriophage of Haemophilus influenzae. 3. Morphology, DNA homology, and immunity properties of HPlcl, S2, and the defective bacteriophage from strain Rd. J Virol. 1973 Apr;11(4):585–591. doi: 10.1128/jvi.11.4.585-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrati-Elizur E., Yosuv D., Shmueli E., Horowitz A. Thymineless death in Bacillus subtilis: correlation between cell lysis and deoxyribonucleic acid breakdown. J Bacteriol. 1974 Jul;119(1):36–43. doi: 10.1128/jb.119.1.36-43.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H. Studies on transformations of Hemophilus influenzae. IV. Linked and unlinked transformations. J Gen Physiol. 1961 Nov;45:205–228. doi: 10.1085/jgp.45.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J. DNA-mediated prophage induction in Bacillus subtilis lysogenic for phi 105c4. J Virol. 1973 Jul;12(1):18–24. doi: 10.1128/jvi.12.1.18-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Kimball R. F., Setlow J. K. Mutation fixation in MNNG-treated Haemophilus influenzae as determined by transformation. Mutat Res. 1974 Jan;22(1):1–14. doi: 10.1016/0027-5107(74)90002-5. [DOI] [PubMed] [Google Scholar]
- Medoff G., Overholt S. Thymineless death in Escherichia coli 15T- and recombinants of 15T- and Escherichia coli K-12. J Bacteriol. 1970 Apr;102(1):213–216. doi: 10.1128/jb.102.1.213-216.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Swartz M. N. Induction of a defective phage and DNA methylation in Escherichia coli 15T-. J Gen Virol. 1969 Jan;4(1):15–27. doi: 10.1099/0022-1317-4-1-15. [DOI] [PubMed] [Google Scholar]
- Michalka J., Goodgal S. H. Genetic and physical map of the chromosome of Hemophilus influenzae. J Mol Biol. 1969 Oct 28;45(2):407–421. doi: 10.1016/0022-2836(69)90115-6. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICARD N., DEVORET R. [Effects of thymine dificiency on the K 12 T lysogenic and 15 T colicinogenic strains of Escherichia coli]. C R Hebd Seances Acad Sci. 1962 Sep 10;255:1417–1419. [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit J. C., Barbee T., Jetton S. Morphological changes in Escherichia coli strain C produced by treatments affecting deoxyribonucleic acid synthesis. J Gen Microbiol. 1967 Oct;49(1):165–173. doi: 10.1099/00221287-49-1-165. [DOI] [PubMed] [Google Scholar]
- Tankersley W. G., Woodward J. M., Brown A. Induction and isolation of a minicell-producing strain of Salmonella typhimurium. Proc Soc Exp Biol Med. 1974 Mar;145(3):802–805. doi: 10.3181/00379727-145-37898. [DOI] [PubMed] [Google Scholar]