Abstract
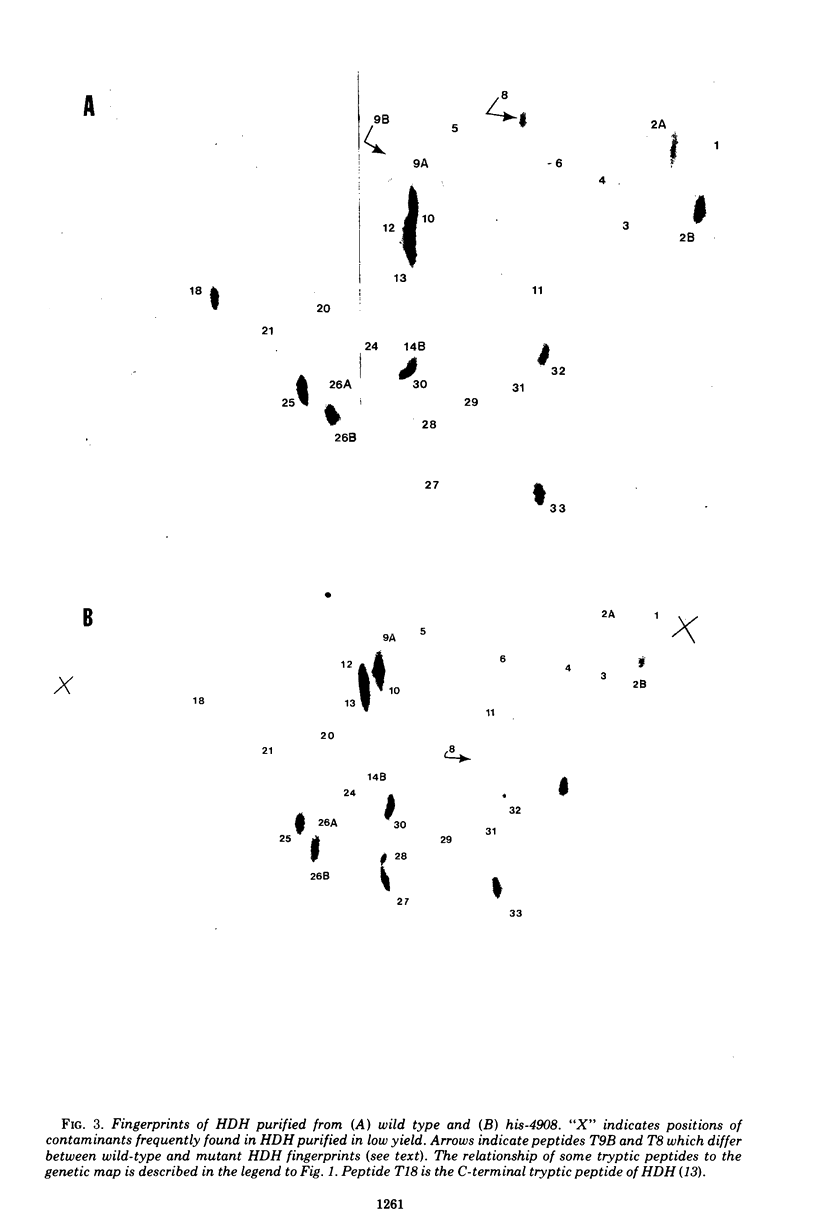
Frameshift mutation hisD497 occurs in the operator-proximal portion of the Salmonella typhimurium gene coding for the dimeric protein, L-histidinol dehydrogenase (HDH). Rare revertants of hisD497 are deletions fusing the hisD gene to the adjacent preceding structural gene, hisG (adenosine 5'-triphosphate-PR transferase). HDH purified from one revertant, hisGD4908, contains subunits of approximately normal molecular weight but with no clearly demonstrable unique amino-terminal sequence. We propose that a combined inactive G-D polypeptide is synthesized and then cleaved at a number of closely juxtaposed sites by endoproteolytic activity. At least some of the resulting fragments then participate in formation of active HDH dimers.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Revertants and secondary arom-2 mutants induced in non-complementing mutants in the arom gene cluster of Neurospora crassa. Genetics. 1974 Aug;77(4):613–626. doi: 10.1093/genetics/77.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Greeb J., Atkins J. F., Loper J. C. Histidinol dehydrogenase (his D) mutants of Salmonella typhimurium. J Bacteriol. 1971 May;106(2):421–431. doi: 10.1128/jb.106.2.421-431.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston L. L. Evidence for proteolytic degradation of histidinol phosphate phosphatase specified by nonsense mutants of the hisB gene of Salmonella typhimurium. J Bacteriol. 1973 Oct;116(1):88–97. doi: 10.1128/jb.116.1.88-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino I., Yourno J. X-ray mutagenesis: base-pair deletion at a frameshift hotspot in Salmonella. J Mol Biol. 1974 May 15;85(2):301–307. doi: 10.1016/0022-2836(74)90365-9. [DOI] [PubMed] [Google Scholar]
- Isono K., Yourno J. Mutation leading to gene fusion in the histidine operon of Salmonella typhimurium. J Mol Biol. 1973 Jun 5;76(4):455–461. doi: 10.1016/0022-2836(73)90484-1. [DOI] [PubMed] [Google Scholar]
- Isono S., Yourno J. Non-suppressible addition frameshift in Salmonella. J Mol Biol. 1974 Jan 25;82(3):355–360. doi: 10.1016/0022-2836(74)90596-8. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Yourno J. Proteolytic release of a histidinol dehydrogenase fragment from the double enzyme histidinol dehydrogenase-imidazolylacetol-phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Apr 10;246(7):2203–2206. [PubMed] [Google Scholar]
- Loper J. C. Histidinol dehydrogenase from Salmonella typhimurium. Crystallization and composition studies. J Biol Chem. 1968 Jun 25;243(12):3264–3272. [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Koshland D. E., Jr Multiple aggregation states of phosphoribosyladenosine triphosphate synthetase. J Biol Chem. 1974 Jul 10;249(13):4119–4126. [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Tanemura S., Yourno J. Frameshift revertant of Salmonella typhimurium producing histidinol dehydrogenase with a sequence of four extra amino acid residues. J Mol Biol. 1969 Dec 28;46(3):459–466. doi: 10.1016/0022-2836(69)90189-2. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J., Appella E., Martin R. G. Purification and composition studies of phosphoribosyladenosine triphosphate:pyrophosphate phosphoribosyltransferase, the first enzyme of histidine biosynthesis. J Biol Chem. 1967 Apr 25;242(8):1760–1767. [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Shiller L. M., Castrilli J. His-linked hydrogen sulfide locus in Salmonella typhimurium. J Bacteriol. 1974 Nov;120(2):902–905. doi: 10.1128/jb.120.2.902-905.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J. Translation and polarity in the histidine operon. 3. The isolation of prototrophic polar mutations. J Mol Biol. 1967 Nov 28;30(1):109–124. doi: 10.1016/0022-2836(67)90247-1. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Gutnick D. L., Margolies M. N., Martin R. G., Rechler M. M., Voll M. J. Relative translation frequencies of the cistrons of the histidine operon. J Mol Biol. 1970 Apr 14;49(1):245–249. doi: 10.1016/0022-2836(70)90391-8. [DOI] [PubMed] [Google Scholar]
- Yourno J. Composition and subunit structure of histidinol dehydrogenase from Salmonella typhimurium. J Biol Chem. 1968 Jun 25;243(12):3277–3288. [PubMed] [Google Scholar]
- Yourno J., Heath S. Nature of the hisD3018 frameshift mutation in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):460–468. doi: 10.1128/jb.100.1.460-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Ino I. Purification and crystallization of histidinol dehydrogenase from Salmonella typhimurium LT-2. J Biol Chem. 1968 Jun 25;243(12):3273–3276. [PubMed] [Google Scholar]
- Yourno J. Nature of the compensating frameshift in the double frameshift mutant hisD3018 R5 of Salmonella typhimurium. J Mol Biol. 1970 Mar;48(3):437–442. doi: 10.1016/0022-2836(70)90056-2. [DOI] [PubMed] [Google Scholar]
- Yourno J. Similarity of cross-suppressible frameshifts in Salmonella typhimurium. J Mol Biol. 1971 Nov 28;62(1):223–231. doi: 10.1016/0022-2836(71)90141-0. [DOI] [PubMed] [Google Scholar]