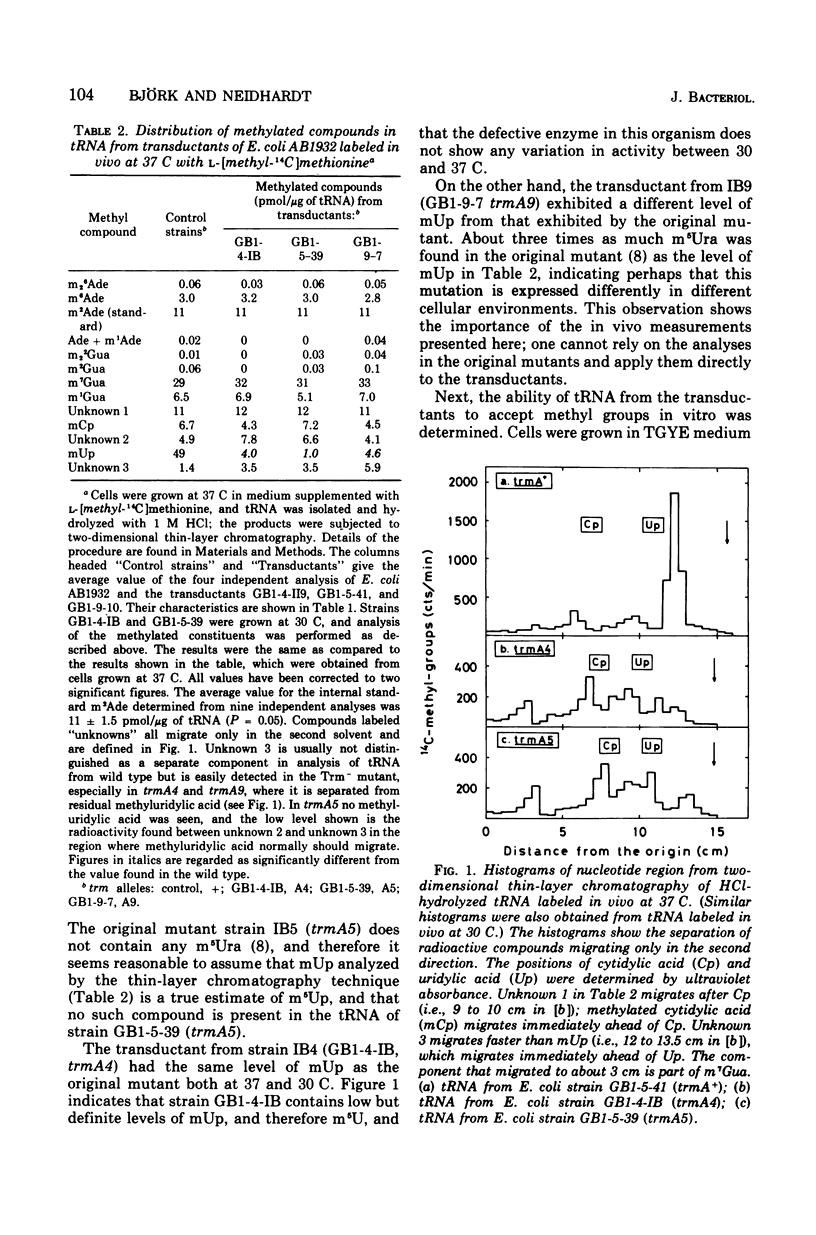
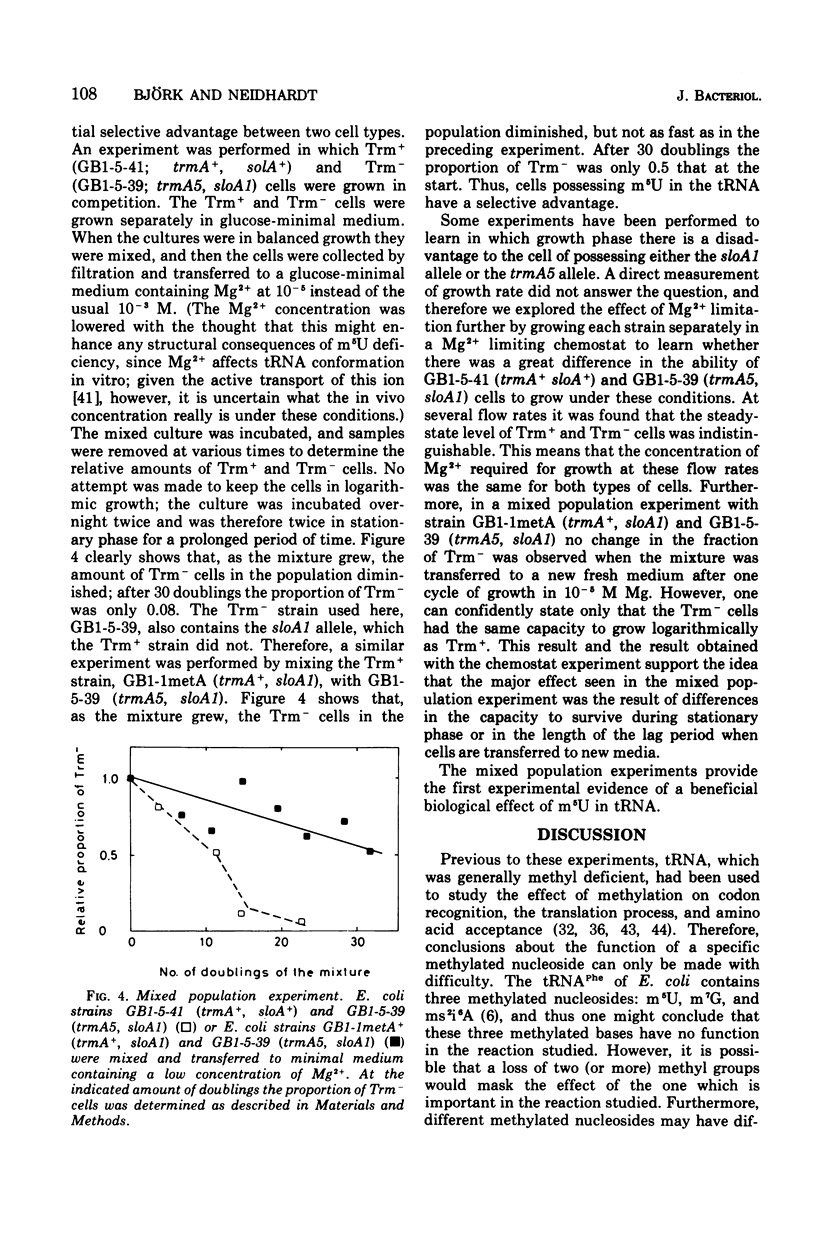
Abstract
Matched pairs of transductant strains differing by the presence of absence of 5-methyluridine (ribothymidine) (m5U) in their transfer ribonucleic acid (tRNA) were used to study the function of this modified nucleoside in Escherichia coli. Ordinary measurements of growth rate in different media revealed no effect of the loss of m5U in tRNA. A gene located close to trmA (the structural cistron for the methyltransferase that produces m5U in tRNA), however, was found to reduce the growth rates significantly, depending on the medium and the temperature of cultivation. Measurement of codon recognition, macromolecular composition, tRNA binding to the ribosome, and the rate of protein chain elongation in vivo indicated no disadvantage caused by the lack of m5U. The regulation of ilv and his operons seemed also to be unaffected by the absence of m5U in the tRNA. In a mixed population experiment, however, cells possessing m5U in their tRNA seemed to have a distinct advantage over cells lacking this modified nucleoside. This experiment provides the first indication of the overall value of m5U in tRNA.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Neidhardt F. C. Unusual valyl-transfer ribonucleic acid synthetase mutant of Escherichia coli. J Bacteriol. 1972 Jan;109(1):307–314. doi: 10.1128/jb.109.1.307-314.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H. H., Schmidt W., Kersten H. Occurrence and biosynthesis of ribothymidine in tRNAs of B. subtilis. FEBS Lett. 1975 Mar 15;52(1):62–65. doi: 10.1016/0014-5793(75)80638-7. [DOI] [PubMed] [Google Scholar]
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- BOMAN H. G., HJERTEN S. "Molecular sieving" of bacterial RNA. Arch Biochem Biophys. 1962 Sep;Suppl 1:276–282. [PubMed] [Google Scholar]
- Barrell B. G., Sanger F. The sequence of phenylalanine tRNA from E. coli. FEBS Lett. 1969 Jun;3(4):275–278. doi: 10.1016/0014-5793(69)80157-2. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Analysis of 5-methyluridine function in the transfer RNA of Escherichia coli. Cancer Res. 1971 May;31(5):706–709. [PubMed] [Google Scholar]
- Björk G. R., Svensson I. Analysis of methylated constituents from RNA by thin-layer chromatography. Biochim Biophys Acta. 1967 Apr 18;138(2):430–432. doi: 10.1016/0005-2787(67)90504-7. [DOI] [PubMed] [Google Scholar]
- Björk G. R. Transductional mapping of gene trmA responsible for the production of 5-methyluridine in transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):92–98. doi: 10.1128/jb.124.1.92-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Hatfield G. W. Autoregulation: a role for a biosynthetic enzyme in the control of gene expression. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2757–2761. doi: 10.1073/pnas.70.10.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Rimerman R. A., Hatfield G. W. Threonine deaminase from Escherichia coli. I. Purification and properties. J Biol Chem. 1973 May 25;248(10):3511–3516. [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Biosynthesis of ribosylthymine in the transfer RNA of Streptococcus faecalis: a folate-dependent methylation not involving S-adenosylmethionine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):528–530. doi: 10.1073/pnas.72.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Partial nucleotide sequence of a prokaryote initiator tRNA that functions in its non-formylated form. Nature. 1974 Nov 8;252(5479):106–109. doi: 10.1038/252106a0. [DOI] [PubMed] [Google Scholar]
- Dube S. K. Recognition of tRNA by the ribosome. A possible role of 5 S RNA. FEBS Lett. 1973 Oct 1;36(1):39–42. doi: 10.1016/0014-5793(73)80332-1. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Osawa S., Miura K. The methyl groups in ribosomal RNA from Escherichia coli. Biochim Biophys Acta. 1966 Dec 21;129(3):519–531. doi: 10.1016/0005-2787(66)90067-0. [DOI] [PubMed] [Google Scholar]
- Johnson L., Hayashi H., Söll D. Isolation and properties of a transfer ribonucleic acid deficient in ribothymidine. Biochemistry. 1970 Jul 7;9(14):2823–2831. doi: 10.1021/bi00816a011. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J. G., Nirenberg M. Ribonucleic acid codons and protein synthesis. 13. RNA codon recognition by deacylated tRNA and aminoacyl-tRNA. J Mol Biol. 1968 Jun 28;34(3):467–480. doi: 10.1016/0022-2836(68)90173-3. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Moore P. B. Methylation of messenger RNA in Escherichia coli. J Mol Biol. 1966 Jun;18(1):38–47. doi: 10.1016/s0022-2836(66)80074-8. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Ofengand J., Henes C. The function of pseudouridylic acid in transfer ribonucleic acid. II. Inhibition of amino acyl transfer ribonucleic acid-ribosome complex formation by ribothymidylyl-pseudouridylyl-cytidylyl-guanosine 3'-phosphate. J Biol Chem. 1969 Nov 25;244(22):6241–6253. [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. Specific recognition of GTpsiC loop (loop IV) of tRNA by 50S ribosomal subunits from E. coli. Nat New Biol. 1973 Dec 5;246(153):132–135. doi: 10.1038/newbio246132a0. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Starr J. L., Sells B. H. Methylated ribonucleic acids. Physiol Rev. 1969 Jul;49(3):623–669. doi: 10.1152/physrev.1969.49.3.623. [DOI] [PubMed] [Google Scholar]
- Svensson I., Isaksson L., Henningsson A. Aminoacylation and polypeptide synthesis with tRNA lacking ribothymidine. Biochim Biophys Acta. 1971 May 13;238(2):331–337. doi: 10.1016/0005-2787(71)90100-6. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S. Ribosome development and the methylation of ribosomal ribonucleic acid. J Bacteriol. 1968 May;95(5):1844–1850. doi: 10.1128/jb.95.5.1844-1850.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söll D. Enzymatic modification of transfer RNA. Science. 1971 Jul 23;173(3994):293–299. doi: 10.1126/science.173.3994.293. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., BERG P. The effect of enzymatically synthesized ribonucleic acid on amino acid incorporation by a soluble protein-ribosome system from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:94–104. doi: 10.1073/pnas.48.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Reinitz E. R., Gefter M. L. Role of modifications in tyrosine transfer RNA. II. Ribothymidylate-deficient tRNA. Arch Biochem Biophys. 1973 Jul;157(1):55–62. doi: 10.1016/0003-9861(73)90389-5. [DOI] [PubMed] [Google Scholar]
- ZAMIR A., HOLLEY R. W., MARQUISEE M. EVIDENCE FOR THE OCCURRENCE OF A COMMON PENTANUCLEOTIDE SEQUENCE IN THE STRUCTURES OF TRANSFER RIBONUCLEIC ACIDS. J Biol Chem. 1965 Mar;240:1267–1273. [PubMed] [Google Scholar]
- Zamenhof S., Eichhorn H. H. Study of microbial evolution through loss of biosynthetic functions: establishment of "defective" mutants. Nature. 1967 Nov 4;216(5114):456–458. doi: 10.1038/216456a0. [DOI] [PubMed] [Google Scholar]


