Abstract
Hydrogel brushes are materials composed of a water-swollen network, which contains polymer chains that are grafted with another polymer. Using a thermally responsive polymer, poly(N-isopropyl acrylamide) (polyNIPAM), as the graft component we are able to maintain the critical solution temperature (Tcrit), independent of the overall composition of the material, at approximately 32°C. The change in swelling at Tcrit is a function of the amount of polyNIPAM in the system. However, there is a much smaller change in the surface contact angles at Tcrit. PolyNIPAM-based materials have generated considerable interest, as ‘smart’ substrates for the culture of cells and here, we show the utility of hydrogel brushes in cell culture. Chondrocytes attached to the hydrogel brushes and yielded viable cell cultures. Moreover, the chondrocytes could be released from the hydrogel brushes without the use of proteases by reducing the temperature of the cultures to below Tcrit to induce a change in the conformation of the polyNIPAM chain at Tcrit. The importance of the crosslink hydrogel component is illustrated by significant changes in cell attachment/cell viability as the crosslink density is changed.
Keywords: poly(N-isopropyl acrylamide), hydrogel, polymer brush, chondrocyte, cell culture
1. Introduction
Thermally responsive polymers, such as poly(N-isopropyl acrylamide) (polyNIPAM), pass through a coil-to-globule transition at a lower critical solution temperature (Tcrit) (Heskins & Guillet 1968). The change from the coil to the globule conformation produces a step change in physical properties. These temperature-dependent changes can be used to prepare stimulus responsive devices that are of enormous interest in tissue engineering (Nishida et al. 2004), controlled delivery (Soppimath et al. 2002) and other areas (Stayton et al. 1995). However, chemically crosslinked hydrogel materials with grafted stimulus responsive chains (hydrogel brushes) do not appear to have been examined. This is surprising because, as grafted chains respond to external stimuli, these materials should be capable of combining the properties of the stimulus responsive brushes with a network structure that maintains its properties above and below Tcrit. We also envisage that below Tcrit, the brush architecture will provide resistance to non-specific protein adsorption in much the same way that grafted poly(ethylene glycol) (PEG) chains provide enhanced biofouling resistance in many biotechnological applications.
One area of application of polyNIPAM materials that is growing in importance is their use as substrata for the culture of cells in tissue engineering (e.g. Yamato et al. 2001; Jeong & Gutowska 2002; Li et al. 2003; Shiroyanagi et al. 2003). The majority of work in this area has been directed at cell expansion in monolayer culture (Yamato et al. 2001; Shiroyanagi et al. 2003). Here, the cells are cultured on polyNIPAM coatings, above Tcrit of polyNIPAM. In this state, many cells adhere and spread and can be cultured to form confluent sheets. However, tissue engineering also requires the rapid expansion of cultured cells in order to generate sufficient numbers to seed a scaffold. Most protocols commonly passage cells using a combination of proteases and ethylenediaminetetraacetic acid (EDTA) to detach a confluent cell sheet from polystyrene tissue culture plates. The addition of animal-derived substances, particularly proteins, is undesirable in the culture of cells for therapeutic purposes owing to the obvious risks of disease transmission. Moreover, there is some evidence that detachment of cells from surfaces with trypsin and EDTA may alter or even damage a cultured cell population. Thus, evidence for trypsin-induced changes in cultured cells has been reported for keratinocytes (Umegaki et al. 2004), epithelial (Reiners et al. 2000) and endothelial cells (Lopes et al. 2001). Although there have been no reports of specific trypsin-induced injury to cultured chondrocytes, it is well known that they lose their phenotype following prolonged expansion and that there are related risks of in vitro growth selection and accelerated ageing (Mayne et al. 1976; von der Mark et al. 1977; Zimmermann et al. 2001; Parsch et al. 2002; Veilleux et al. 2004). While it is difficult to separate the factors responsible for phenotypic changes, the evidence summarized above would suggest that proteases used in cell passaging play a significant role.
Cells for therapeutic applications should ideally be expanded without the use of substances that have the potential to transmit disease and alter cell phenotype and condition. Thermoresponsive surfaces offer a promising alternative to conventional methods and therefore deserve careful investigation. In addition, recently published data suggest that the low-temperature removal of endothelial cells from plasma-polymerized polyNIPAM does less damage to the extracellular matrix proteins that are left behind than conventional methods (Canavan et al. 2005). To date, the use of polyNIPAM as a thermally responsive culture system has concentrated on systems in which the globular (hydrophobic) form of polyNIPAM provides an acceptable surface for cell adherence and proliferation. In most variants of the technique, the material lying below the polyNIPAM layer is impervious. However, we considered that a hydrogel layer lying beneath the polyNIPAM layer would allow nutrients and growth factors to perfuse to both sides of the cells. In this paper, we examine the feasibility of culturing bovine chondrocytes on stimulus responsive hydrogel brushes and releasing these cells by thermal switching through the globule-to-coil transition. Previous work has shown that polyNIPAM surfaces modified with a RGD-containing peptide could be used to culture and release chondrocytes (Kim et al. 2002). However, these random hydrophilic modifications to the polyNIPAM sequences also increase the Tcrit towards the optimized temperatures for cell cultures and this is expected to have a detrimental effect on cell adhesion during culture.
In the system reported here, above Tcrit, polyNIPAM adopts a globular conformation and cell adhesion is controlled by protein adsorption to both the hydroxylic and the polyNIPAM components. However, below Tcrit, polyNIPAM adopts an open chain conformation and the material is converted into a hydrogel brush. The open chain hydrogel brush state is expected to be highly resistant to protein adsorption and non-fouling in the same way that PEGylated surfaces are non-fouling and non-adherent.
2. Results
We chose to use poly(2,3 propandiol-1-methacrylate) (polyGMA) as the hydrogel component. This material has been shown by us and others (Haigh et al. 2000, 2002) to be generally resistant to protein adsorption and cell adhesion. However, we believe that this is the first report of the thermally induced modification of a hydroxylic hydrogel. The only previous example of a similar stimulus responsive material appears to be a pH-responsive hydrogel brush recently reported by Ohsedo et al. (2004) and we are unaware of any other examples for the use of similar surfaces for the culture of mammalian cells.
Stimulus responsive hydrogel brushes were made using a macromonomer approach, i.e. an oligo(NIPAM) was prepared by radical polymerization in the presence of a carboxylic acid functional thiol chain transfer agent (Rimmer et al. 1996) (see figure 1 for the synthesis process). This material was then converted to a macromonomer (MNIPAM) by reaction with chloromethyl styrene. The network brushes were then produced by terpolymerization of MNIPAM with a difunctional monomer, ethandiol dimethacrylate (EDMA) and 2,2-dimethyl-[1,3]dioxolan-4-yl methylene methacrylate (GMAc). The polymeric residues of GMAc were then hydrolysed to liberate the hydroxylic repeat unit, 2,3 propandiol-1-methacrylate (GMA). The use of GMAc in these preparations rather than direct copolymerization with GMA is preferable owing to difficulties in the purification of the latter. Distillation of GMA often results in spontaneous polymerization, whereas GMAc can be easily and reproducibly purified by vacuum distillation.
Figure 1.
Scheme showing the synthetic route to a thermal stimulus responsive hydrogel brush.
The synthesis described above provides relatively densely crosslinked gels. Assuming complete efficiency of the crosslinking process, we estimate that, on average, the number of monomers per crosslink, N, decreases (with increasing MNIPAM concentration) from eight to three in the most densely crosslinked series (7 wt% EDMA), although the series with only 1 wt% EDMA added has values of N decreasing from 61 to 25.
The Tcrit of polyNIPAM sections of the hydrogel brushes can be determined by observing the peak temperature of endotherm associated with the desolvation and coil collapse of the polyNIPAM segments. Differences between the structure of water in these hydrogel brushes and conventional copolymer networks were qualitatively illustrated by our failure to observe an endotherm using DSC from the Tcrit process in most of the examples of a set of random terpolymer hydrogels. The latter were produced by radical polymerization of EDMA, NIPAM and GMA. Figure 2 shows the Tcrits of the hydrogel brushes and clearly shows that the brush architecture produces a constant value of the Tcrit regardless of the overall composition of the material. The use of the peak of endotherm in the DSC technique probably over estimates the Tcrit when compared with other methods. However, the results given here clearly show the independence of Tcrit of the polyNIPAM segments from both the ratio of the monomer repeat units and the degree of swelling, which is set by the concentration of EDMA in the monomer feed. The onset temperatures of the endotherm were 32°C in all cases.
Figure 2.
Tcrits of the hydrogel brushes (determined from DSC measurements). EDMA in monomer feed (wt%) filled circles, 1; filled squares, 4; filled triangles, 7 (data points fitted as a visual aid only).
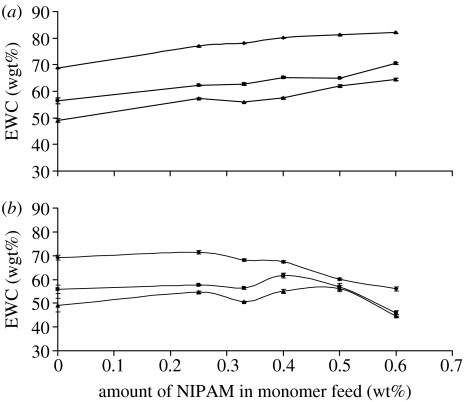
Hydrogel brushes display unusual temperature-dependent swelling behaviour. Figure 3a,b gives the equilibrium water contents (EWC=mass of water/mass of water and polymer) above and below Tcrit. Below Tcrit, the polyNIPAM segments act to increase the EWC and increasing the fraction of polyNIPAM in the system increases the EWC. In addition, as in conventional swollen networks, increasing the crosslink density (by increasing the fraction of EDMA in the monomer feed) decreases the EWC. However, above Tcrit, the polyNIPAM segments act as hydrophobic units and the EWC decreases as the fraction polyNIPAM increases. One important consequence of this behaviour is that, at Tcrit, the change in EWC increases as the fraction of polyNIPAM increases. Thus, there are larger changes in the degree of swelling at Tcrit for materials containing larger fractions of polyNIPAM than those containing smaller fractions. We have also observed this effect in random copolymer hydrogels produced from terpolymerization of EDMA, NIPAM and GMA. However, the random copolymer architecture of these materials has a significant effect on Tcrit, which is increased by incorporation of GMA to values that are impractical for cell culture. The effect of this phenomenon (the change in Tcrit with terpolymer composition) was that we were unable to find suitable conditions for successful cell culture on these random terpolymer hydrogels. Thus, one of the important advantages of the use of hydrogel brush architecture is that we are able to alter the degree of swelling, above and below Tcrit, independent of Tcrit.
Figure 3.
EWCs of the network brushes (a) below Tcrit (20°C) and (b) above Tcrit (60°C) (data points fitted as a visual aid only).
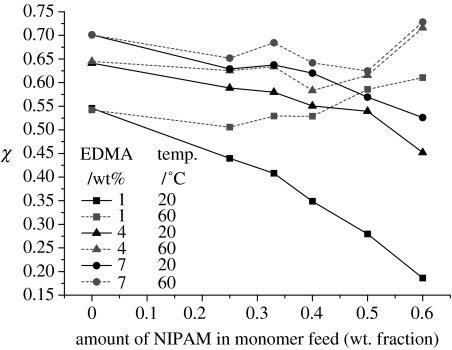
In order to gain some physical understanding of the behaviour of the gels and the effect of the polyNIPAM grafted chains, we can estimate a Flory–Huggins interaction parameter, Χ. Because Χ is a measure of immiscibility, greater values indicate more water exclusion. To obtain Χ, we use simple mean field theory, which has long been used to analyse the swelling behaviour of polymer gels, and with some success, particularly in the case of solvent swollen gels where the gel exhibits a more uniform swelling behaviour appropriate for a mean field analysis. Although there have been many modifications to mean field theory, the most commonly used are the affine Flory and the phantom models (Russ et al. 2003, and references therein). The phantom model is generally more successful for the analysis of solvent swelling because it allows the crosslinks some mobility. The affine model, however, predicts a uniform (affine) change in the distance between crosslinks, which is inappropriate for such heterogeneous systems. The interaction parameter is then calculated from the following equation:
| (2.1) |
where Γ=0.5 according to the phantom model for a crosslinking functionality of 4; ϕ is the volume fraction of gel; ϕs is the volume fraction of gel at its preparation state; and the lattice volume is normalized to the size of a water molecule. In fact, an analysis based on the affine Flory model does not change the results significantly. The results are plotted in figure 4 for the different crosslinking densities as a function of polyNIPAM concentration. We note that the environmental response of gel is the greatest for the smallest cross-link densities. The effect of the polyNIPAM is clearly visible with the greatest difference in Χ for the larger crosslink densities. The synthetic procedure applied here means that the crosslinking density changes even for a constant wt% of EDMA because we have added polyNIPAM at the expense of GMA.
Figure 4.
Χ calculated from mean field theory plotted as a function of polyNIPAM weight fraction above and below Tcrit.
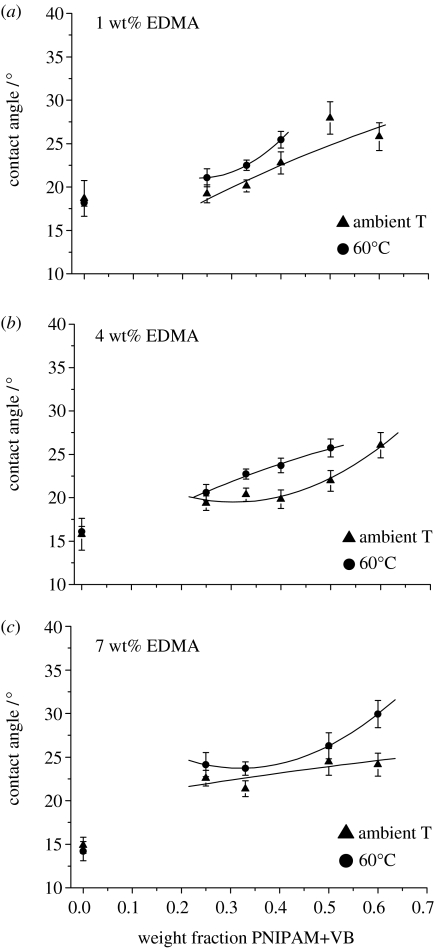
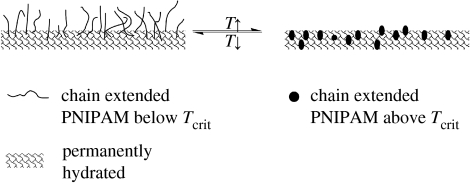
Contact angle measurements can be used to obtain a relative measure of surface energy. Previous work has shown that there are large changes (10–50°) in the contact angle as polyNIPAM materials are progressed through Tcrit (Takei et al. 1994; Liang et al. 2000; Ista et al. 2001; Gupta & Khandeka 2003; de las Heras Alarcon et al. 2005). However, there are also reports of quite small changes (less than 10°) in the contact angle at Tcrit of surface grafted polyNIPAM (Akiyama et al. 2004; Cheng et al. 2005). The contact angle was determined above and below Tcrit using the captive bubble technique with a goniometer. The results are displayed in figure 5. Within each set of materials, the contact angle increases as the materials are progressed through Tcrit. However, while the increases are consistent with the concept of a switch from a hydrophilic to a more hydrophobic state at Tcrit, the differences are relatively small (less than 10°). Thus, it is likely that as the temperature is increased through Tcrit, in aqueous media, the polymer segments rearrange to present the most hydrophilic interface possible. This means that the hydrophilic fully solvated interface, which is presented below Tcrit, is replaced by a hydrogel interface composed mainly of the polyGMA segments, which are hydrophobically modified with the globular polyNIPAM segments. The proposed structural reorganization that occurs around Tcrit is illustrated in figure 6.
Figure 5.
Contact angles of the hydrogel brushes measured below Tcrit (20°C) and above Tcrit (60°C) (data points fitted as a visual aid only).
Figure 6.
Schematic of the coil-to-globule transition in a hydrogel brush. At T<Tcrit, the thermally responsive chains are solvated and extended into the continuous phase. At T>Tcrit, the thermally responsive chains are desolvated and are in a globular hydrophobic state. The crosslink network remains swollen above Tcrit.
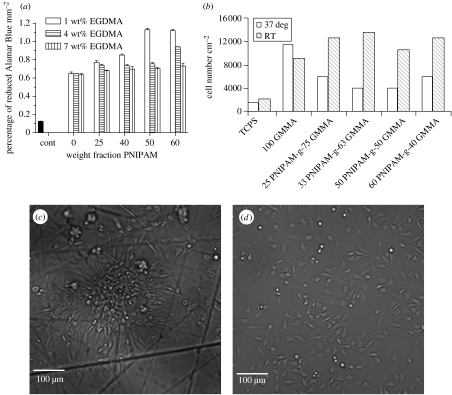
Bovine chondrocytes were cultured on the hydrogel brushes. Figure 7a shows the viability of these cultures as assessed by the degree of reduction of the vital dye, Alamar Blue, by the cultured chondrocytes, which is a measure of cellular activity and which, in turn, is indicative of the number of cells in the culture. The data are expressed as a percentage of the reduced form of the dye per square millimetre of culture surface. The reduction of Alamar Blue observed by chondrocytes cultured on ultra-low attachment tissue culture plastic is also shown in figure 7a. In these cultures, the chondrocytes could not attach to the culture surface and the level of activity is a reflection of the numbers of cells loosely associated with the surface, which had not been removed during media changes during the 5-day culture period. This group is representative of the rate of Alamar Blue reduction which would be observed if the chondrocytes did not attach to the polymer surfaces.
Figure 7.
(a) Effect of the weight fraction of PNIPAM and wt% EGDMA in the graft copolymer networks on the cellular activity of bovine chondrocytes after 5 days of culture on the hydrogels compared to chondrocyte activity of cells remaining on ultra-low attachment tissue culture plastic (designated control in the figure). (b) Effect of decreasing the incubation temperature from 37°C to room temperature (i.e. below the LCST of the hydrogels) on cell attachment to the hydrogels containing 1 wt% EGDMA. The open columns show the numbers of chondrocytes found in the culture medium (i.e. cells unattached to the culture substrate) at 37°C. The hatched columns show the numbers of chondrocytes found in the culture medium after cooling the cultures to room temperature. Results are given standardized to the area of the hydrogels or TCPS. (c) and (d) Phase contrast micrographs of chondrocytes showing cellular attachment to cultured on 60NIPAM-g-40GMMA-r−4EGDMA (figure 7c) and TCPS (figure 7d). The cells were fixed in situ with p-formaldehyde after 48 h in culture to permit observation by light microscopy.
The first observation to be made from these results is that the chondrocytes are attached to all the hydrogel surfaces, since all the cell/polymer cultures showed greater reduction of Alamar Blue compared with the ultra-low attachment tissue culture plastic control (figure 7a). Further, addition of the polyNIPAM segments to the hydrogels increased the reduction of Alamar Blue (figure 7a). Addition of 25–60 wt% PNIPAM to gels crosslinked with 1 wt% ethylene glycol dimethacrylate (EGDMA) significantly (p<0.001) increased the reduction of Alamar Blue indicating a larger cell number/activity. Addition of PNIPAM segments to gels crosslinked with 4 or 7 wt% EDMA did not show such a large effect on the rate of Alamar Blue reduction although addition of the higher concentrations of PNIPAM (50–60 wt%) significantly showed enhanced Alamar Blue reduction compared with gels having no PNIPAM (figure 7a). The increase in Alamar Blue reduction shows that the addition of PNIPAM segments caused either an increase of cell activity on the polymer surface or, more likely, the result of the NIPAM segments enabling increased numbers of cells to attach and/or proliferate to the polymers. This latter hypothesis is supported by the increase in the number of cells released from the hydrogels at room temperature as the amount of polyNIPAM in the hydrogels is increased. The increase in cell attachment might possibly have been mediated by increased protein adsorption to the polymer surface as the amount of polyNIPAM is increased.
From figure 7a, it can be seen that an increase in the content of EDMA from 1 to 7%, which increases the crosslink density of the network, has the effect of decreasing the amount of reduced Alamar Blue and hence reflects a reduction in the number of cells attached to the polymer surface. This apparent decrease in cell numbers/viability with increasing crosslink density is an indication of the role that the underlying hydrogel plays in the culture of cells on these surfaces. Thus, decreasing crosslink density should increase the exchange of nutrients and waste products to and from the cells and this in turn should increase the viability of the culture and aid the rate of cell proliferation. Thus, the most successful chondrocyte cultures were carried out at the lowest crosslink density (i.e. hydrogel brushes prepared with 1 wt% EDMA) and the highest concentration of polyNIPAM.
The thermoresponsive nature of polyNIPAM in the synthesized copolymer networks meant that they were very sensitive to temperature changes and their opacity made the microscopic examination of the polymer/chondrocyte cultures difficult. Hence, microscopic examination was only possible on cell cultures fixed with p-formaldehyde. Phase contrast micrographs of cells cultured on tissue culture plastic substrate (TCPS) and a material composed of polyNIPAM (60 parts by weight), GMA (40 parts by weight) and EDMA (4 parts by weight) are shown in figure 7c.
Once the chondrocytes had been cultured on the networks, they were released by cooling the polymer to room temperature and the numbers of free cells determined by direct cell counting. Figure 7b shows the results of these experiments carried out on the set of hydrogel brushes that contained 1% w/w of EDMA. The first observation to be made is that when comparing the numbers of cells found in the culture medium at 37°C (indicative of the numbers of chondrocytes not attached to the culture substrate and present as suspension cultures), fewer chondrocytes were observed in the culture medium when cells were cultured on standard TCPS than when any of the networks were used. The highest number of chondrocytes found in the medium at 37°C was in cultures of cells with poly(GMA-co-EDMA). Clearly, this substrate without modification was a poor surface for the culture of chondrocytes; most of the cells did not adhere to the substrate and were present as a cell suspension in the culture medium. Modification with polyNIPAM grafts increased chondrocyte attachment as shown by a reduced number of cells present in the culture medium at 37°C (i.e. a reduction in unattached cells). Cooling the polymer/chondrocyte cultures to room temperature released the cells from the gel surface, so that increased numbers of cells were detected in the samples of culture media taken at room temperature. Microscopical examination suggested that no cells remained attached to the surfaces of materials following this temperature change. In contrast, cooling had little effect on the adhesion of chondrocytes cultured on TCPS. No significant changes in the number of cells in the culture medium were observed on lowering the temperature of cell cultures on TCPS from 37°C to room temperature.
3. Discussion and conclusions
Hydrogel brushes are a relatively under-studied class of materials. They are similar to the many examples of surface-grafted brushes that have appeared in the literature. However, since the underlying substrate (the hydrogel) is crosslinked and swollen, they should be expected to have very different properties to brushes formed onto non-swollen surfaces. The hydrogel brushes described here are thermally responsive and can be used as stimulus responsive substrates for cell culture. It was not possible to culture chondrocyte cells on analogous random copolymer networks. One of the motivations for examining the use of these materials as substrata for cell culture was the possibility of culturing cells at relatively high degrees of swelling, while maintaining the temperature of the thermal coil-to-globule transition. These relatively high degrees of swelling above the critical coil-to-globule transition, compared to similar polyNIPAM networks and grafted surfaces, should allow for improved diffusion of nutrients and matrix components, such as growth factors. This concept was demonstrated by the observation that increasing the crosslink density of the hydrogel decreased the viability of the culture. The swollen nature of the hydrogel phase dominated the changes in contact angle as the polyNIPAM phase progressed through Tcrit. In each material, the contact angle increases consistently as the temperature of the measurement was increased from 20–60°C. However, although the change is significant, the increase in contact angle is small. Interestingly, although we observed differences in the magnitude of change in contact angle, at Tcrit, the magnitude of the change could not be correlated to the ability of the materials to release cells into the media as the temperature was decreased. Much larger effects are observed in the degree of swelling obtained above and below Tcrit. Above Tcrit, the polyNIPAM segments are in a globular and hydrophobic form and act to hydrophobically modify the hydrogel phase. Therefore, increasing the fraction of polyNIPAM produced a decrease in swelling. On the other hand, below Tcrit, the polyNIPAM segments are extended into the solution and as the fraction of polyNIPAM increases, swelling increases. Thus, the change in swelling at Tcrit became more pronounced as the fraction of polyNIPAM increased. Kwon & Matsuda (2006) recently reported that increasing the NIPAM fraction in poly(NIPAM-block-PEG) linear block copolymers improved the viability of chondrocytes. In the present work, we also found that it was not possible to culture bovine chondrocytes on poly(EDMA-co-GMA) hydrogels unless they were modified by terpolymerization with the oligo(NIPAM). Chondrocytes cultured on these materials adhered to the substrate above Tcrit, but they were released below Tcrit. Hence, these results suggest that the main factors driving the release of the cells are probably increased swelling and a switch to the more mobile open-chain brush architecture at Tcrit rather than the apparent changes in the contact angle at Tcrit. This release of the cultured cells, and presumably the release of the associated extracellular matrix, is similar to the well-known ability of grafted water-swollen polymer brushes (e.g. PEG brushes) to produce non-biofouling surfaces. We also observed a clear effect of the crosslink density on viability of the chondrocyte cultures. Increasing crosslink density decreases the degree of swelling. If we compare materials containing the same fraction of polyNIPAM, we can conclude that decreased swelling (increased cross-link density) decreased cell viability. This decrease in cell activity could possibly be associated with changes in the permeation characteristics of the materials, i.e. lower degrees of swelling may result in lower rates of permeation of nutrients, waste products and biomolecules. However, further work is necessary to test this hypothesis.
4. Methods
4.1 Synthesis of poly(N-isopropyl acrylamide) macromonomers
Radical polymerization of N-isopropylacrylamide monomer (NIPAM; 100 g, Aldrich, UK; recrystallized from hexane) in ethanol (200 cm3, Fisher, UK; Laboratory Grade) in the presence of α,α′-azo-bis-isobutyronitrile (AIBN; 1 g, Aldrich, UK; recrystallized from methanol) and mercaptopropionic acid (3 g, Aldrich, UK; distilled) gave a polyNIPAM with a carboxylic acid end group. The monomer solution was placed into the reaction vessel and purged with dry nitrogen for 2 h. Polymerization was initiated at 60°C under a dry nitrogen atmosphere for a further 24 h. The polymer was dried, dissolved in 1,4-dioxane (Aldrich, UK; spectrophotometric grade), filtered and then precipitated into diethyl ether (Fisher Scientific, UK; Laboratory Grade). The precipitate was washed three times with clean diethyl ether before being dried overnight under vacuum at 40°C. This process was repeated until removal of impurities and residual solvent was confirmed by 1H NMR spectroscopy (–SCH2CH2*COOH 2.4–2.6 p.p.m., –SCH2*CH2COOH 2.65–2.8 p.p.m., (CH3)2CH*– 3.85–4.05 p.p.m.). End group analysis using the 1H gave a Mn=12 000 g mol−1. MALDI TOF mass spectrometry (using a Micromass TofSpec 2E instrument with 2,5-dihydroxybenzoic acid as the matrix) showed that the end group mass was, as expected, m/z 106.9. Each ion peak in the molecular weight distribution was separated by the repeat mass of polyNIPAM (m/z 113.1).
The resultant polyNIPAM was dissolved in de-ionized water and caesium carbonate (Aldrich, UK) was added to the solution under agitation until evolution of carbon dioxide gas ceased. Then the solution was dried using vacuum evaporation. The oligo(NIPAM) was dissolved in acetone (Fisher, UK; Laboratory Grade) and 1 g Cs2CO3 was added. The solution was purged with dry nitrogen for 2 h. An excess of vinyl benzyl chloride was added into the reaction vessel. The mixture was heated to 50°C for 24 h under a dry nitrogen atmosphere. The reacted solution was dried and the residue was dissolved in 1,4-dioxane (Aldrich, UK; spectrophotometric grade) and filtered. The product was precipitated into diethyl ether and washed three times with diethyl ether before being dried under vacuum at 40°C. This process was repeated until removal of impurities and residual solvent was confirmed by 1H NMR spectroscopy. The styryl end groups were observed in the 1H NMR spectrum (5.05–5.11 p.p.m., =CH2 5.20–5.23 and 5.25–5.28 p.p.m., –CH=5.68–5.70 and 5.74–5.79 p.p.m., C6H4 7.1–7.4 p.p.m.). MALDI TOF mass spectrometry showed that the end group mass was, as expected, m/z 223.3.
Poly(NIPAM-graft-GMA-co-EDMA) networks were synthesized from the radical polymerization of GMAc, MNIPAM macromonomer and EDMA. The monomer mixture and AIBN dissolved in 2-propanol were added to a polymerization mould, which consisted of a 2 mm thick polytetrafluoroethylene gasket sandwiched between two pieces of commercially available poly(ethylene terephthalate) film (Hifi Industrial Film Ltd, UK; PMX 727, 100 μm, no slip). This in turn was sandwiched between two glass plates, the mould being held together by metal paper clips. Polymerization was carried out at 60°C for 24 h. Upon completion, the crosslinked copolymer network was removed from the mould and washed in distilled IPA five times, over a period of 3 days. The densities required to convert weight fraction to volume fraction for the thermodynamic calculations of equation (2.1) are 1.051 g cm−3 (EGDMA, Aldrich information), 1.312 g cm−3 (PGMA, measured using a density bottle), and 1.13 and 1.17 g cm−3 for polyNIPAM above and below Tcrit, respectively (Seelenmeyer et al. 2001). The volume fraction of gel in the preparation state is ca 0.7, where small variations from sample to sample have no significant effect on the results calculated from equation (2.1).
4.2 Measurements
The water contact angle of copolymer networks was determined using the captive bubble technique with a Ramé-Hart contact angle goniometer equipped with a Fostec AC1 light source. EWCs were determined gravimetrically.
4.3 Isolation and culture of bovine chondrocyte
Chondrocytes were isolated from all cartilages as described previously (Crawford & Dickinson 2004). Full-thickness hyaline cartilage was harvested from bovine metacarpophalangeal joints of adult animals (18–24 months) within 4 h of slaughter. Chondrocytes were released by sequential proteolytic digestion at 37°C for 30 min in trypsin (0.25% in PBS) followed by incubation for 18–22 h with 0.2% bacterial collagenase (Sigma, UK) in basic culture medium (Dulbecco's modified eagles medium (high-glucose formula containing GLUTAMAX-1), containing 10 mM HEPES, 10% FBS, non-essential amino acids, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin sulphate). The isolated cells were seeded in tissue culture plates (3×104 cells cm2) and cell numbers expanded for two to three passages in basic medium containing 10 ng ml−1 FGF-2 (PreproTech, UK). All reagents were from Sigma Aldrich, UK unless stated otherwise.
4.4 Chondrocyte culture on thermoresponsive copolymers
Thermoresponsive copolymer networks were stored in distilled isopropyl alcohol before use. For cell culture, the polymers were washed in de-ionized water, 8 mm discs cut and placed (1/well) into ultra-low attachment 24-well tissue culture plates (Corning, UK). The plates were incubated at 37°C for 5 h in 1 ml of well-distilled water. The water was replaced with pre-warmed basic culture medium and incubated overnight at 37°C. The medium was then removed from each well and 2 ml of chondrocyte suspension in basic culture medium (pre-warmed to 37°C) was added to each well. The plates were incubated at 37°C. Throughout all the stages of culture manipulation, the temperature of polymer and medium was kept between 32 and 37°C.
4.5 Measurement of cell detachment
Chondrocytes were seeded onto the polymer as above and incubated at 37°C for 24 h. A sample of medium was taken from each culture at 37°C to determine the numbers of cells unattached to the polymer surfaces at 37°C. Cell detachment was determined as follows. The cell cultures were allowed to cool to room temperature for 15 min. To determine the numbers of cells which had detached from the polymer surface, samples of culture medium were gently removed from each well after the cooling period. The number of chondrocytes in the samples of culture media taken before and after cooling was then determined using a haemocytometer.
4.6 Statistical analysis
Statistical significance was determined by two-way ANOVAR followed by a Bonferroni post-test using GraphPad v. 4.
4.7 Measurement of cellular activity by Alamar Blue
Cell viability of chondrocyte cultures was monitored by calorimetric assay (Fields & Lancaster 1993) using Alamar Blue according to the manufacturer's instructions (Biosource Europe S.A., Belgium). Polymers were seeded with chondrocytes in the same manner described earlier. After 5 days of culture, the culture medium was gently removed (while keeping the cultures at 37°C) and immediately replaced with pre-warmed fresh culture medium at 37°C containing Alamar Blue. The polymer/cell cultures were incubated for 2 h at 37°C. Cell-free polymers were also incubated in the same way. 200 μl samples of culture medium were then removed from all the wells and the levels of reduced and oxidized Alamar Blue determined spectrophotometrically. Cell-free polymers were also incubated with Alamar Blue in the same way to determine any dye reduction in the absence of cells. Cell viability was calculated as the percentage of reduced Alamar Blue produced by the chondrocytes and expressed as the percentage of reduced Alamar Blue per square millimetre of culture surface.
References
- Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–5511. doi: 10.1021/la036139f. [DOI] [PubMed] [Google Scholar]
- Canavan H.E, Cheng X, Graham D.J, Ratner B.D, Castner D.G. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J. Biomed. Mater. Res. A. 2005;75:1–13. doi: 10.1002/jbm.a.30297. [DOI] [PubMed] [Google Scholar]
- Cheng X, et al. Surface chemical and mechanical properties of plasma-polymerized N-isopropylacrylamide. Langmuir. 2005;21:7833–7841. doi: 10.1021/la050417o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Dickinson S.C. Chondrocyte isolation, expansion and culture on polymer scaffolds. In: Hollander A.P, Hatton P.V, editors. Biopolymer methods in tissue engineering. Humana Press; Totowa, NJ: 2004. pp. 147–157. [DOI] [PubMed] [Google Scholar]
- de las Heras Alarcon C, Farhan T, Vicky L, Osborne V.L, Huck Wilhelm T.S, Alexander C. Bioadhesion at micro-patterned stimuli-responsive polymer brushes. J. Mater. Chem. 2005;15:2089–2094. [Google Scholar]
- Fields R.D, Lancaster M.V. Duel attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993;11:48–50. [PubMed] [Google Scholar]
- Gupta K.C, Khandeka K. Temperature-responsive cellulose by Ceric(IV) ion-initiated graft copolymerization of N-isopropylacrylamide. Biomacromolecules. 2003;4:758–776. doi: 10.1021/bm020135s. [DOI] [PubMed] [Google Scholar]
- Haigh R, Fullwood N, Rimmer S. Synthesis and properties of amphiphilic networks 1: the effect of hydration and polymer composition on the adhesion of immunoglobulin-G to Poly(laurylmethacrylate-stat-glycerolmethacrylate-stat-ethylene-glycol-dimethacrylate) networks. Biomaterials. 2000;21:735–739. doi: 10.1016/S0142-9612(99)00245-8. [DOI] [PubMed] [Google Scholar]
- Haigh R, Fullwood N, Rimmer S. Synthesis and properties of amphiphilic networks 2: a differential scanning calorimetric study of poly(dodecyl methacrylate-stat-2,3 propandiol-1-methacrylate-stat-ethandiol dimethacrylate) networks and adhesion and spreading of dermal fibroblasts on these materials. Biomaterials. 2002;23:3509–3516. doi: 10.1016/S0142-9612(02)00081-9. [DOI] [PubMed] [Google Scholar]
- Heskins M, Guillet J.E. Solution properties of poly(N-isopropylacrylamide) J. Macromol. Sci. 1968;A2:1441–1455. [Google Scholar]
- Ista L.K, Mendez S, Perez-Luna V.H, Lopez G.P. Synthesis of poly(N-isopropylacrylamide) on initiator-modified self-assembled monolayers. Langmuir. 2001;17:2552–2555. [Google Scholar]
- Joeng B, Gutowska A. Lessons from nature: stimuli-responsive polymers and their applications. Trends Biotechnol. 2002;20:305–310. doi: 10.1016/S0167-7799(02)01962-5. [DOI] [PubMed] [Google Scholar]
- Kim M.R, Jeong J.H, Park T.G. Swelling induced detachment of chondrocytes using RGD-modified poly(N-isopropylacrylamide) hydrogel beads. Biotechnol. Prog. 2002;18:495–500. doi: 10.1021/bp020287z. [DOI] [PubMed] [Google Scholar]
- Kwon I.K, Matsuda T. Photo-iniferter-based thermoresponsive block copolymers composed of poly(ethylene glycol) and poly(N-isopropylacrylamide) and chondrocyte immobilization. Biomaterials. 2006;27:986–995. doi: 10.1016/j.biomaterials.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Li F, et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl Acad. Sci. USA. 2003;100:15 346–15 351. doi: 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Rieke P.C, Liu J, Fryxell G.E, James S, Young J.S, Mark H, Engelhard M.H, Alford K.L. Surfaces with reversible hydrophilic/hydrophobic characteristics on crosslink poly(N-isopropylacrylamide) hydrogels. Langmuir. 2000;16:8016–8023. doi: 10.1021/la0010929. [DOI] [Google Scholar]
- Lopes A.A.B, Peranovich T.M.S, Maeda N.Y, Bydlowski S.P. Differential effects of enzymatic treatments on the storage and secretion of von Willebrand factor by human endothelial cells. Thromb. Res. 2001;101:291–297. doi: 10.1016/S0049-3848(00)00401-1. [DOI] [PubMed] [Google Scholar]
- Mayne R, Vail M.S, Mayne P.M, Miller E.J. Changes in type of collagen synthesised as clones of chick chondrocytes grow and eventually lose division capacity. Proc. Natl Acad. Sci. USA. 1976;73:1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- Ohseda Y, Takashina R, Gong J.P, Osada Y. Surface friction of hydrogels with well-defined polyelectrolyte brushes. Langmuir. 2004;20:6549–6555. doi: 10.1021/la036211+. [DOI] [PubMed] [Google Scholar]
- Parsch D, Brummendorf T.H, Richter W, Fellenberg J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002;46:2911–2916. doi: 10.1002/art.10626. [DOI] [PubMed] [Google Scholar]
- Reiners J.J, Mathieu P, Okafor C, Putt D.A, Lash L. Depletion of cellular glutathione by conditions used for the passaging of adherent cultured cells. Toxicol. Lett. 2000;115:153–163. doi: 10.1016/S0378-4274(00)00189-2. [DOI] [PubMed] [Google Scholar]
- Rimmer S, Mohd Ramli A.N, Lefèvre S. Preparation of polystyrene-poly(styrene-g-N-isopropylacrylamide) core-shell particles: copolymerization of oligo(N-isopropylacrylamide) macromonomers and styrene onto polystyrene seed particles and stability of the resultant particles. Polymer. 1996;37:4135–4139. doi: 10.1016/0032-3861(96)00253-4. [DOI] [Google Scholar]
- Russ T, Brenn R, Geoghegan M. Equilibrium swelling of polystyrene networks by linear polystyrene. Macromolecules. 2003;36:127–141. doi: 10.1021/ma0211885. [DOI] [Google Scholar]
- Seelenmeyer S, Deike I, Rosenfeldt S, Norhausen C, Dingenouts N, Ballauff M, Narayanan T, Lindner P. Small-angle X-ray and neutron scattering studies of the volume phase transition in thermosensitive core-shell colloids. J. Chem. Phys. 2001;114:10 471–10 478. doi: 10.1063/1.1374633. [DOI] [Google Scholar]
- Shiroyanagi Y, Yamato M, Yamazaki Y, Toma H, Okano T. Transplantable urothelial cell sheets harvested noninvasively from temperature-responsive culture surfaces by reducing temperature. Tissue Eng. 2003;9:1005–1012. doi: 10.1089/107632703322495646. [DOI] [PubMed] [Google Scholar]
- Soppimath K.S, Aminabhavi T.M, Dave A.M, Kumbar S.G, Rudzinski W.E. Stimulus–responsive “smart” hydrogels as novel drug delivery systems. Drug Dev. Ind. Pharm. 2002;28:957–974. doi: 10.1081/DDC-120006428. [DOI] [PubMed] [Google Scholar]
- Stayton P.S, Shimoboji T, Long C, Chilkoti A, Ghen G, Harris J.M, Hoffman A.S. Control of protein–ligand recognition using a stimuli-responsive polymer. Nature. 1995;378:472–474. doi: 10.1038/378472a0. [DOI] [PubMed] [Google Scholar]
- Takei Y.G, Aoki T, Sanui K, Ogata N, Sakurai Y, Okano T. Dynamic contact angle measurement of temperature-responsivesurface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules. 1994;27:6163–6166. doi: 10.1021/ma00099a035. [DOI] [Google Scholar]
- Umegaki R, Masahiro K-O, Taya M. Assessment of cell detachment and growth potential of human keratinocyte based on observed changes in individual cell area during trypsinization. Biochem. Eng. J. 2004;17:49–55. doi: 10.1016/S1369-703X(03)00124-4. [DOI] [Google Scholar]
- Veilleux N.H, Yannas I.V, Spector M. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng. 2004;10:119–127. doi: 10.1089/107632704322791763. [DOI] [PubMed] [Google Scholar]
- von der Mark K, Gauss V, von der Mark H, Mueller P. Relationship between cell shape and type of collagen synthesized as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- Zimmermann T, et al. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture—primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72–76. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]