Abstract
Three enzymes with L- and one enzyme with D-aminopeptidase (EC 3.4.11; alpha-aminoacyl peptide hydrolase) activity have been separated from each other and partially purified from Bacillus subtilis 168 W.T., distinguished with respect to their molecular weights and catalytic properties, and studied in relation to the physiology of this bacterium. One L-aminopeptidase, designated aminopeptidase I, has a molecular weight of 210,000 +/- 20,000, is produced early in growth, and hydrolyzes L-alanyl-beta-naphthylamide most rapidly. Another, designated aminopeptidase II, molecular weight 67,000 +/- 10,000, is also produced early in growth and hydrolyzes L-lysyl-beta-naphthylamide most rapidly. A third, aminopeptidase III, molecular weight 228,000 +/- 20,000, is produced predominantly in early stationary phase and most efficiently utilizes L-alpha-aspartyl-beta-naphthylamide as substrate. The synthesis of aminopeptidase III in early stationary phase suggests that selective catabolism of peptides occurs at this time, perhaps related to the cessation of growth or the onset of early sporulation-associated events. A D-aminopeptidase which hydrolyzes the carboxyl-blocked dipeptide D-alanyl-D-alanyl-beta-naphthylamide (as well as D-alanyl-beta-naphthylamide and D-alanyl-D-alanyl-D-alanine) has also been identified, separated from aminopeptidase II, and purified 170-fold. D-Aminopeptidase, molecular weight 220,000 +/- 20,000, is localized predominantly in the cell wall and periplasm of the organism. This evidence and the variation of the activity during the growth cycle suggest an important function in cell wall or peptide antibiotic metabolism.
Full text
PDF


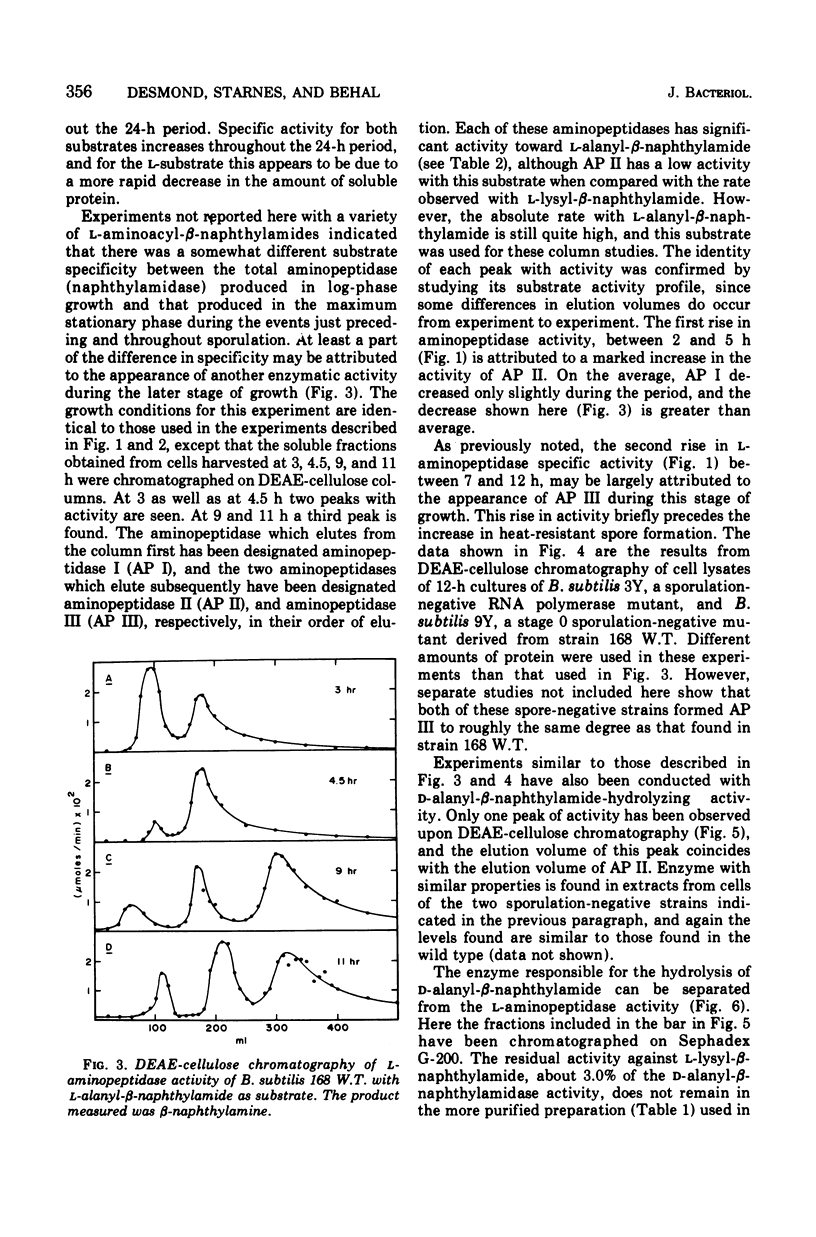



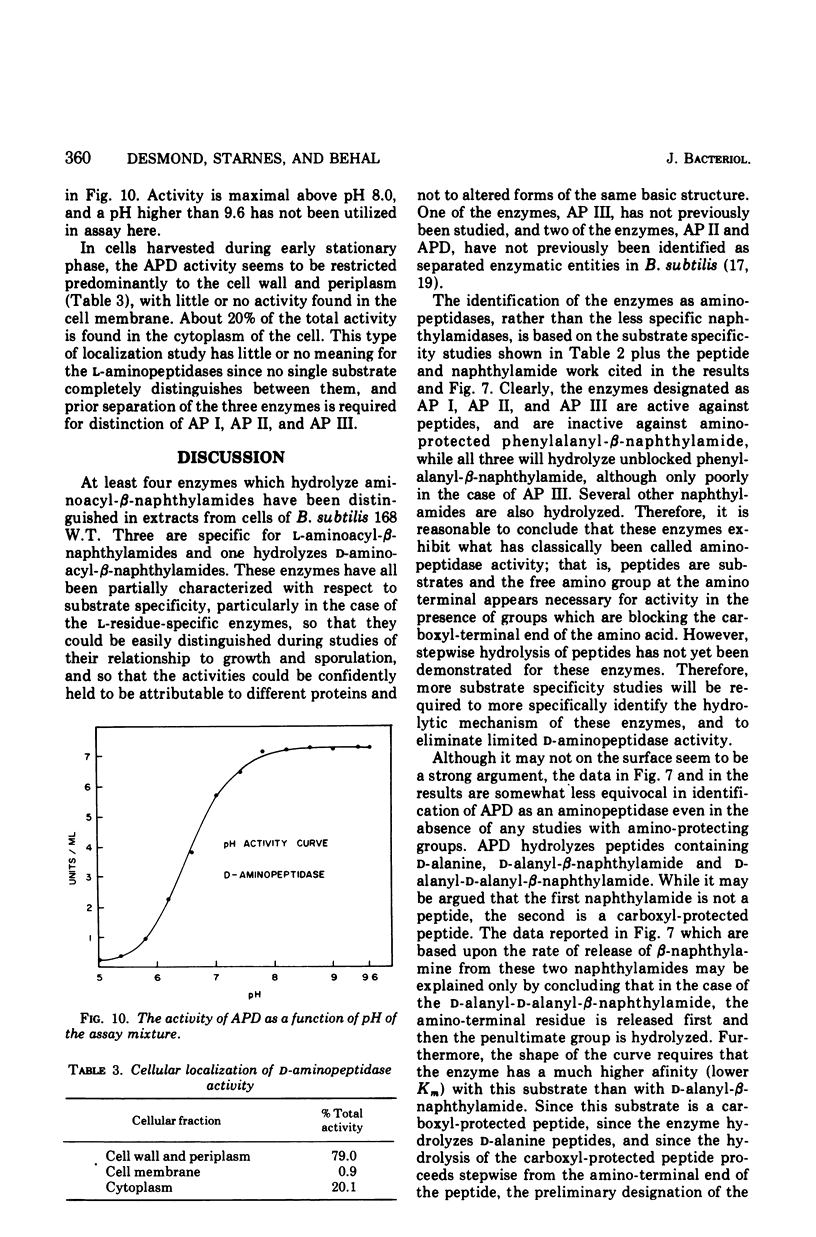



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J. P., Millet J. Etude d'une L-leucyl-beta-naphtylamide hydrolase en relation avec la sporulation chez Bacillus megaterium. C R Acad Sci Hebd Seances Acad Sci D. 1965 Nov 15;261(20):4274–4277. [PubMed] [Google Scholar]
- Behal F. J., Asserson B., Dawson F., Hardman J. A study of human tissue aminopeptidase components. Arch Biochem Biophys. 1965 Aug;111(2):335–344. doi: 10.1016/0003-9861(65)90194-3. [DOI] [PubMed] [Google Scholar]
- Behal F. J., Carter R. T. Naphthylamidases of Sarcina lutea. Can J Microbiol. 1971 Jan;17(1):39–45. doi: 10.1139/m71-007. [DOI] [PubMed] [Google Scholar]
- Behal F. J., Cox S. T. Arylamidase of Neisseria catarrhalis. J Bacteriol. 1968 Oct;96(4):1240–1248. doi: 10.1128/jb.96.4.1240-1248.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. W., Jr, Behal F. J. Human liver aminopeptidase. Role of metal ions in mechanism of action. Biochemistry. 1974 Jul 30;13(16):3227–3233. doi: 10.1021/bi00713a005. [DOI] [PubMed] [Google Scholar]
- Hall F. F., Kunkel H. O., Prescott J. M. Multiple proteolytic enzymes of Bacillus licheniformis. Arch Biochem Biophys. 1966 Apr;114(1):145–153. doi: 10.1016/0003-9861(66)90315-8. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Peterson J. A., Yousten A. A. Unique biochemical events in bacterial sporulation. Annu Rev Microbiol. 1970;24:53–90. doi: 10.1146/annurev.mi.24.100170.000413. [DOI] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little G. H., Behal F. J. Hydrolysis of di- and oligopeptides by human liver arylamidase. Proc Soc Exp Biol Med. 1971 Mar;136(3):954–957. doi: 10.3181/00379727-136-35404. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrin P. A., Hofsten B. V. Purification and metal ion activation of an aminopeptidase (aminopeptidase II) from Bacillus stearothermophilus. Biochim Biophys Acta. 1974 May 20;350(1):13–25. doi: 10.1016/0005-2744(74)90198-3. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Huff E., Cole R. M., Theodore T. S. Cellular location of degradative enzymes in Staphylococcus aureus. J Bacteriol. 1974 Dec;120(3):1012–1016. doi: 10.1128/jb.120.3.1012-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley P. S., Behal F. J. Amino acid- -naphthylamide hydrolysis by Pseudomonas aeruginosa arylamidase. J Bacteriol. 1971 Nov;108(2):809–816. doi: 10.1128/jb.108.2.809-816.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- Starnes W. L., Behal F. J. A human liver aminopeptidase. The amino acid and carbohydrate content, and some physical properties of a sialic acid containing glycoprotein. Biochemistry. 1974 Jul 30;13(16):3221–3227. doi: 10.1021/bi00713a004. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- Wagner F. W., Chung A., Ray L. E. Characterization of the aminopeptidase from Bacillus subtilis as an extracellular enzyme. Can J Microbiol. 1972 Dec;18(12):1883–1891. doi: 10.1139/m72-293. [DOI] [PubMed] [Google Scholar]


