Abstract
If bacteria are incapable of forming and incorporating proteins into the cytoplasmic membranes in all phases of the cell cycle, then not all cells from an asynchronous culture should be capable of growth when switched to a new carbon and energy source whose metabolism requires new membrane function. The transfer of an inducible culture to low lactose provides such a situation since the cells cannot grow unless galactoside permease can function to concentrate the lactose internally. From such experiments, it was concluded that the Y gene product of the lac operon is synthesized, incorporated, and can start functioning in active transport, at any time throughout the bulk of the cell cycle. Not only were the lags before growth re-ensued much shorter than would be expected if the membrane transport capability could only be developed in a small portion of the cycle, but brief pulses of a gratuitous inducer shortened the lags much further. Three types of Escherichia coli ML 30 culture were studied: cells that had exhausted the limiting glucose; cells taken directly from glucose-limited chemostats; and a washed suspension of highly catabolite repressed cells from cultures grown in high levels of glucose and gluconate. The growth studies reported here were performed on-line with a minicomputer. They represent at least an order of magnitude increase in accuracy in estimating growth parameters over previous instrumentation.
Full text
PDF

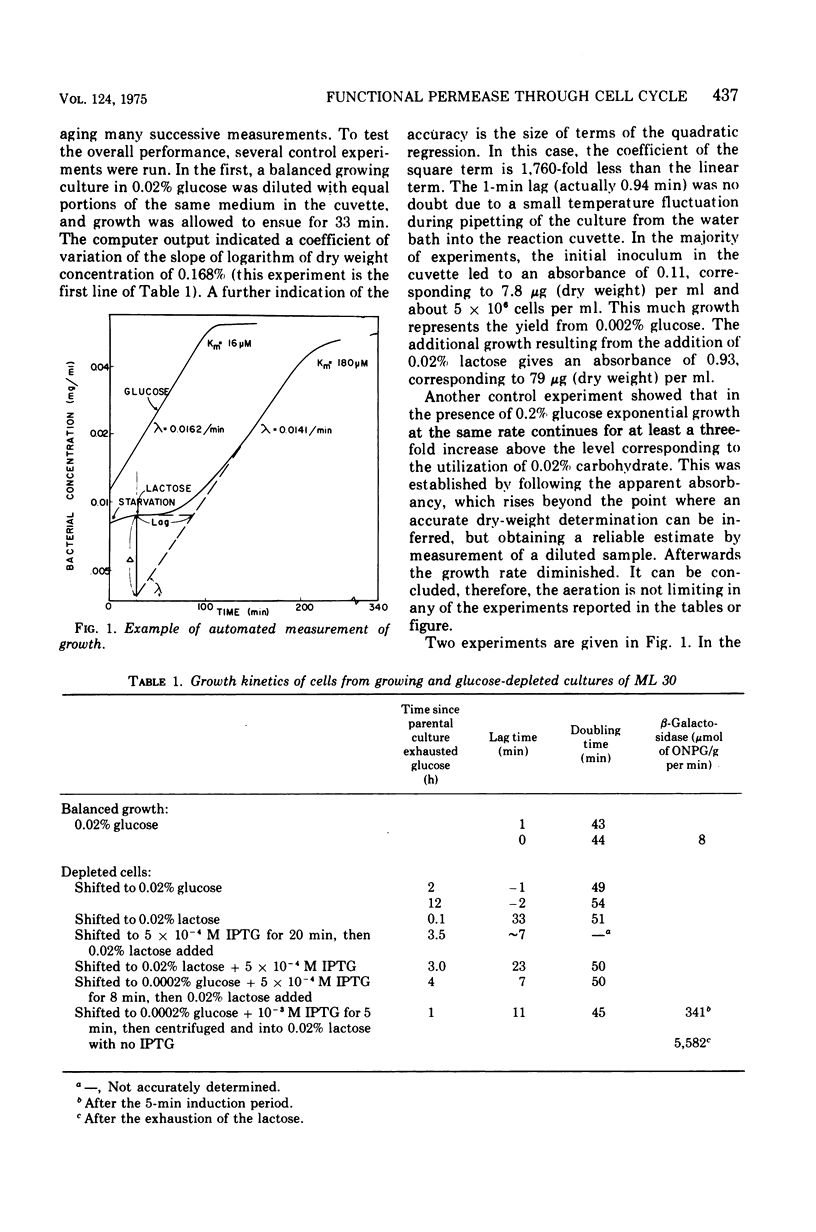


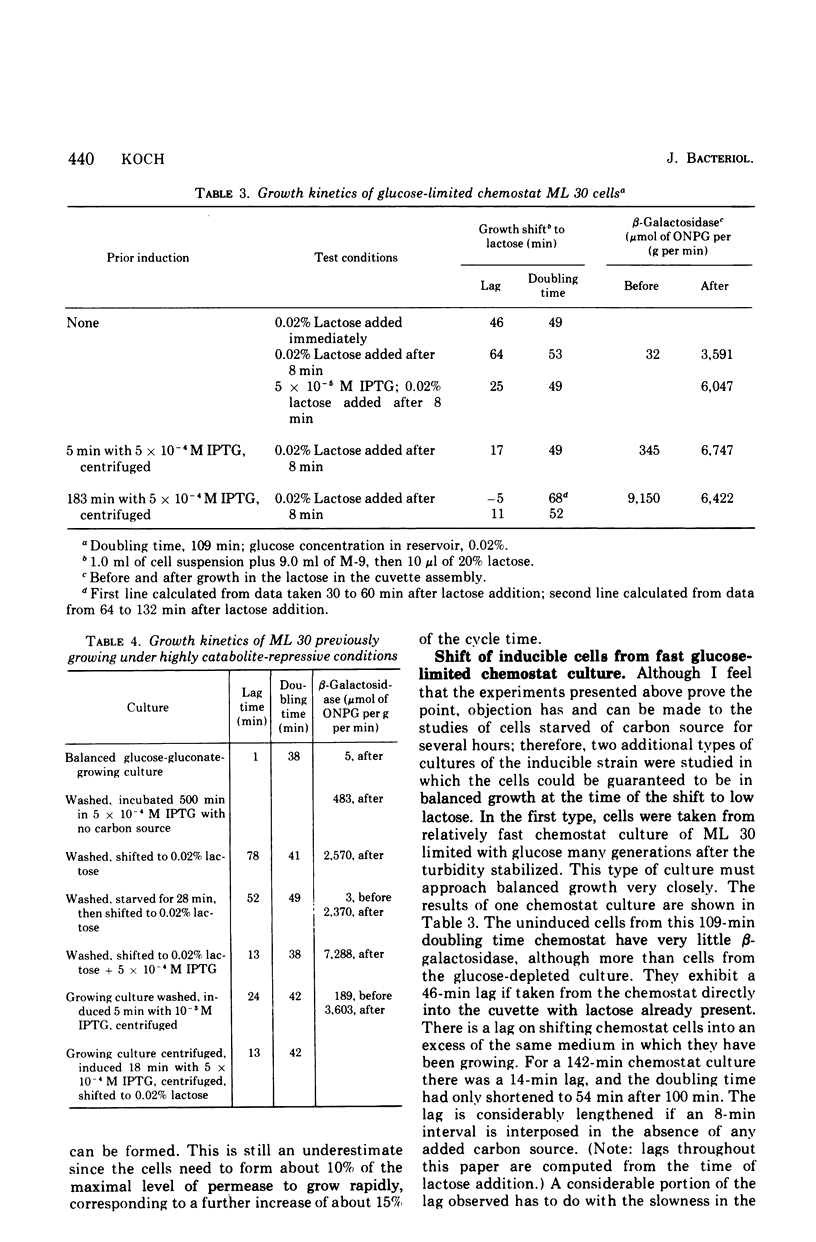




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Tomkins G. M. Sequential transcription of the genes of the lactose operon and its regulation by protein synthesis. J Biol Chem. 1966 Oct 10;241(19):4434–4443. [PubMed] [Google Scholar]
- COHEN S. S., ARBOGAST R. Chemical studies in host-virus interactions; a comparison of some properties of three mutant pairs of bacterial viruses, T2r and T2r, T4r and T4r, T6r and T6r. J Exp Med. 1950 Jun 1;91(6):619–636. doi: 10.1084/jem.91.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Analysis of the differentiation and of the heterogeneity within a population of Escherichia coli undergoing induced beta-galactosidase synthesis. J Bacteriol. 1959 Nov;78:613–623. doi: 10.1128/jb.78.5.613-623.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Origin and sequence of chromosome replication in Escherichia coli B-r. J Bacteriol. 1968 May;95(5):1634–1641. doi: 10.1128/jb.95.5.1634-1641.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Rickenberg H. V. Catabolite repression in Escherichia coli: the role of glucose 6-phosphate. Biochem Biophys Res Commun. 1967 Nov 17;29(3):303–310. doi: 10.1016/0006-291x(67)90453-6. [DOI] [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. lac Repressor-operator interaction. VI. The natural inducer of the lac operon. J Mol Biol. 1972 Aug 28;69(3):397–408. doi: 10.1016/0022-2836(72)90253-7. [DOI] [PubMed] [Google Scholar]
- Kepes A. Sequential transcription and translation in the lactose operon of Escherichia coli. Biochim Biophys Acta. 1967 Mar 29;138(1):107–123. doi: 10.1016/0005-2787(67)90591-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Local and non-local interactions of fluxes mediated by the glucose and galactoside permeases of Escherichia coli. Biochim Biophys Acta. 1971 Oct 12;249(1):197–215. doi: 10.1016/0005-2736(71)90097-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Constancy of uptake during the cell cycle in Escherichia coli. Biophys J. 1968 Dec;8(12):1401–1412. doi: 10.1016/S0006-3495(68)86562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Control of cell growth in bacteria: experiments with thymine starvation. J Bacteriol. 1971 Feb;105(2):472–476. doi: 10.1128/jb.105.2.472-476.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Freedman M. L., Silver S. Potassium uptake in synchronous and synchronized cultures of Escherichia coli. Biophys J. 1971 Oct;11(10):787–797. doi: 10.1016/S0006-3495(71)86254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Unsaturated fatty acid synthesis is not required for induction of lactose transport in Escherichia coli. J Biol Chem. 1974 Feb 10;249(3):724–731. [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., WILLIAMSON J. P., HOOD J. R., Jr, KOCH A. L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962 Nov;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- Shen B. H., Boos W. Regulation of the -methylgalactoside transport system and the galatose-binding protein by the cell cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1481–1485. doi: 10.1073/pnas.70.5.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubitschek H. E. Linear cell growth in Escherichia coli. Biophys J. 1968 Jul;8(7):792–804. doi: 10.1016/s0006-3495(68)86521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]


