Abstract
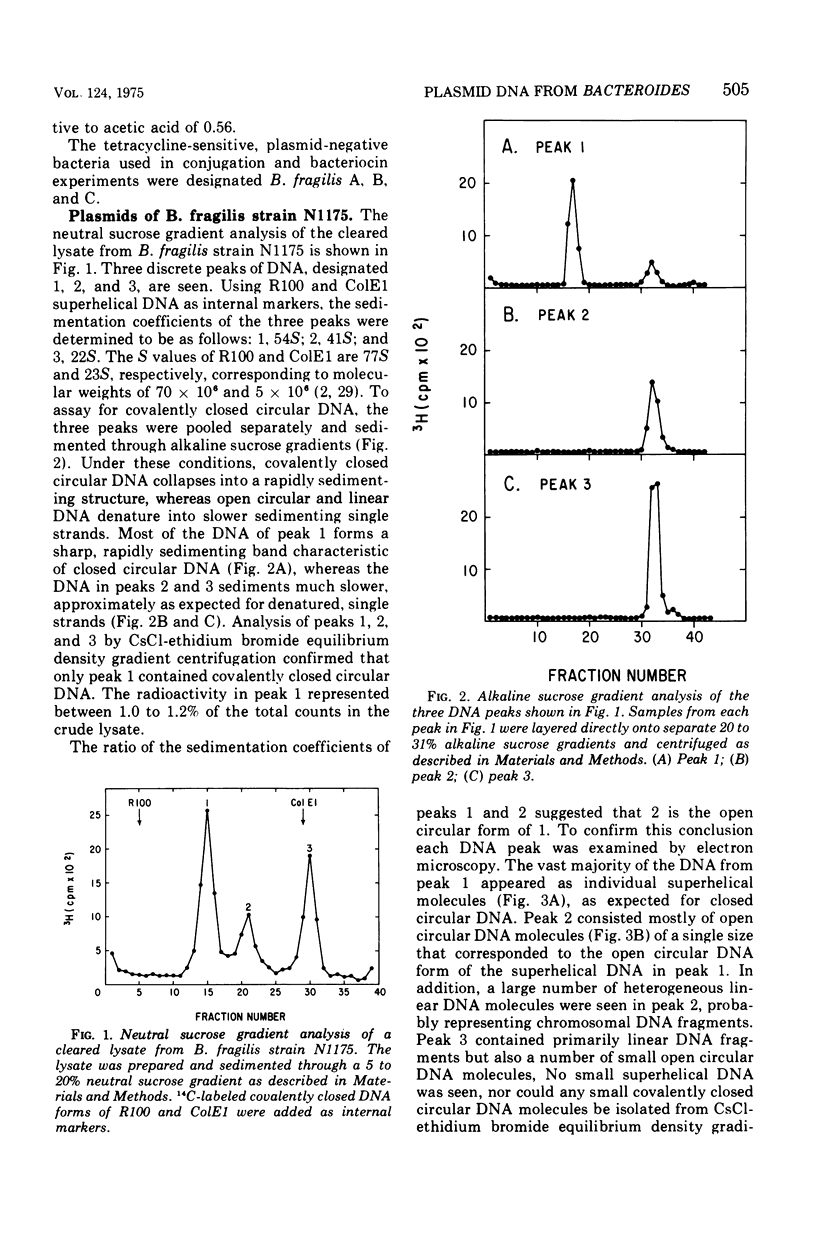
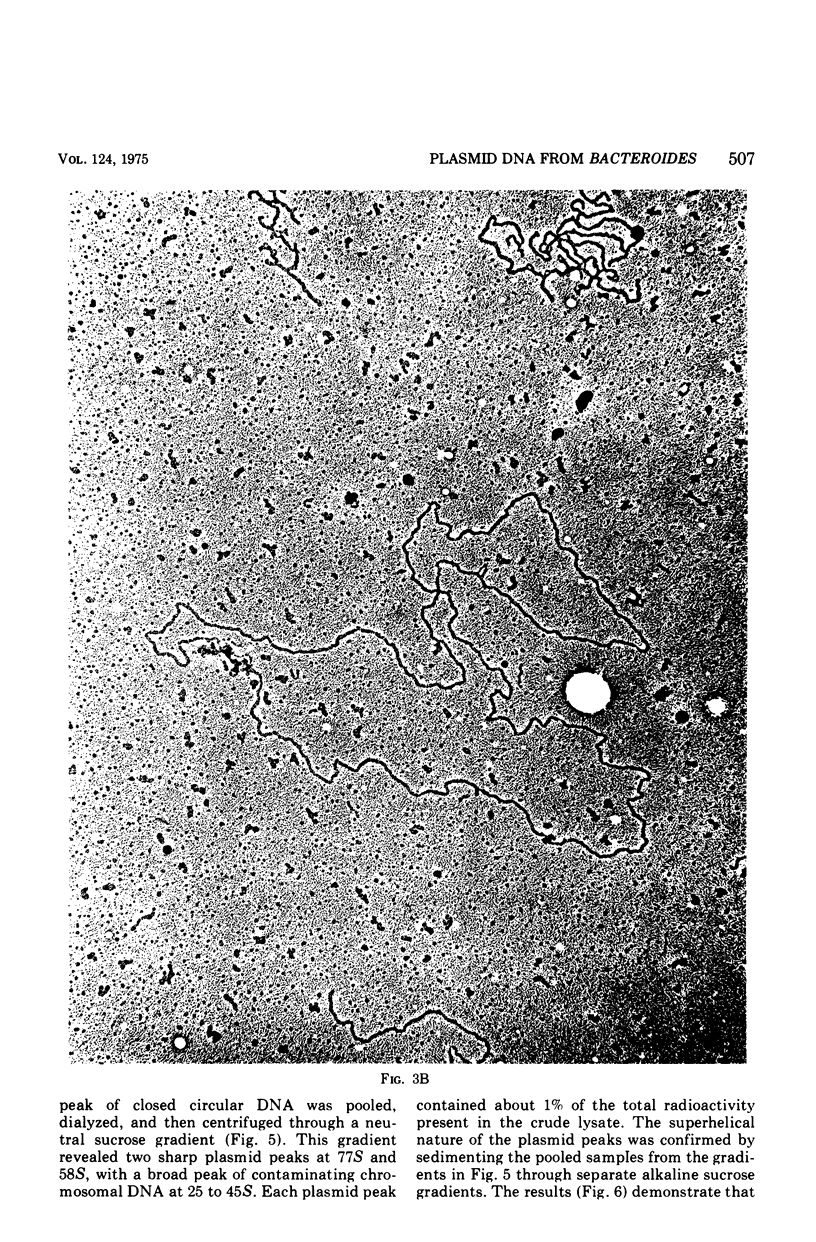
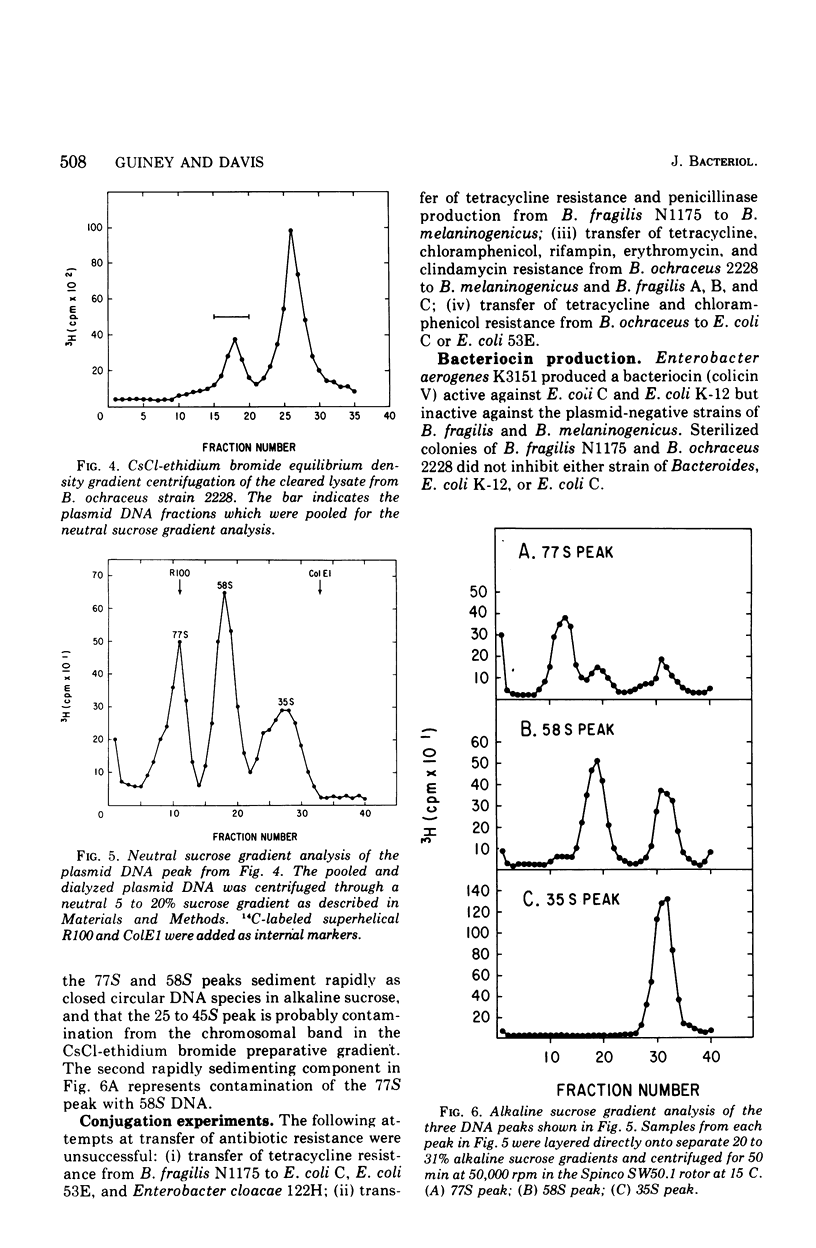
Two clinical isolates of Bacteroides contained covalently closed circular deoxyribonucleic acid (DNA) as shown by sedimentation in an alkaline sucrose gradient, CsCl ethidium bromide equilibrium centrifugation, and electron microscopy. Bacteriodes fragilis N1175 contained a homogeneous species of plasmid DNA with a molecular weight of 25 x 10(6). Bacteroides ochraceus 2228 contained two distinct, covalently closed circular DNA elements. The larger cosedimented with the covalently closed circular DNA form of the R plasmid, R100, corresponding to a molecular weight of 70 x 10(6); the smaller sedimented as a 58S molecule with a calculated molecular weight of 25 x 10(6). The roles of these plasmids are unknown. Neither strain transferred antibiotic resistance to plasmid-negative Bacteroides or Escherichia coli, and neither produced bacteriocins active against other Bacteroides or sensitive indicator strains of E. coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Sykes R. B. Characterisation of a -lactamase obtained from a strain of Bacteroides fragilis resistant to -lactam antibiotics. J Med Microbiol. 1973 May;6(2):201–206. doi: 10.1099/00222615-6-2-201. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Siemienski J. S. The influence of bacteriocins on resistance to infection by gram-negative bacteria. II. Colicin action, transfer of colicinogeny, and transfer of antibiotic resistance in urinary infections. J Clin Invest. 1968 Aug;47(8):1763–1773. doi: 10.1172/JCI105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Helinski D. R. Heterogeneous circular DNA elements in vegetative cultures of Bacillus megaterium. Proc Natl Acad Sci U S A. 1969 Oct;64(2):592–599. doi: 10.1073/pnas.64.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Davis C. E., Anandan J. The evolution of r factor. A study of a "preantibiotic" community in Borneo. N Engl J Med. 1970 Jan 15;282(3):117–122. doi: 10.1056/NEJM197001152820302. [DOI] [PubMed] [Google Scholar]
- Davis C. E., Hunter W. J., Ryan J. L., Braude A. I. Simple method for culturing anaerobes. Appl Microbiol. 1973 Feb;25(2):216–221. doi: 10.1128/am.25.2.216-221.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S. Cultivation of anaerobic intestinal bacteria. J Pathol Bacteriol. 1967 Oct;94(2):417–427. doi: 10.1002/path.1700940223. [DOI] [PubMed] [Google Scholar]
- Drasar B. S., Hill M. J., Shiner M. The deconjugation of bile salts by human intestinal bacteria. Lancet. 1966 Jun 4;1(7449):1237–1238. doi: 10.1016/s0140-6736(66)90242-x. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- Inselburg J. R factor deoxyribonucleic acid in chromosomeless progeny of Escherichia coli. J Bacteriol. 1971 Feb;105(2):620–628. doi: 10.1128/jb.105.2.620-628.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. Plasmid in Bacillus pumilus and the enhanced sporulation of plasmid-negative variants. J Bacteriol. 1973 Jul;115(1):291–298. doi: 10.1128/jb.115.1.291-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Episome-carried surface antigen K88 of Escherichia coli. I. Transmission of the determinant of the K88 antigen and influence on the transfer of chromosomal markers. J Bacteriol. 1966 Jan;91(1):69–75. doi: 10.1128/jb.91.1.69-75.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G., Veo G., Braude A. I. Bacteroides penicillinase. J Bacteriol. 1968 Oct;96(4):1437–1438. doi: 10.1128/jb.96.4.1437-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Stiffler P. W., Keller R., Traub N. Isolation and characterization of several cryptic plasmids from clinical isolates of Bacteroides fragilis. J Infect Dis. 1974 Nov;130(5):544–548. doi: 10.1093/infdis/130.5.544. [DOI] [PubMed] [Google Scholar]




