Abstract
The overlapping distribution of spinal neurons activated with either pudendal sensory nerve or pelvic nerve stimulation was examined in the female rat using c-fos immunohistochemistry. Pudendal sensory nerve stimulation resulted in a significant increase in fos-positive cells in the ipsilateral dorsal horn and bilaterally in the medial, lateral and intermediate gray of L5-S1. Pelvic nerve stimulation resulted in significant increases of c-fos immunoreactive nuclei in the ipsilateral dorsal horn, lateral and intermediate gray and bilaterally in the medial gray of L5-S1. Co-distribution of fos immunoreactive nuclei with the vesicular glutamate transporters (VGlut2 and VGlut3) and neurokinin I receptors were found in distinct regions of the dorsal horn, medial and lateral gray. Specific areas in the medial dorsal horn, dorsal gray commissure, laminae VI and X and dorsal lateral gray were activated after stimulation of the pudendal sensory and pelvic nerves, suggesting these areas contain spinal neurons that receive both somatomotor and visceral inputs and are part of the intraspinal circuit that regulates sexual and voiding function.
Keywords: c-fos, spinal interneurons, urogenital, glutamate, NK1
1. Introduction
The autonomic and somatic peripheral nerves that innervate the pelvic organs are important in mediating and coordinating responses seen during female reproductive behavior and micturition. Sensory inputs from the pudendal nerve relay information relevant to mating that is received during anogenital investigation and mounting behavior and this input can trigger sexual arousal and lordosis [1,31,33,45,47]. The pelvic nerve conveys sensory information during vaginocervical stimulation, can modulate lordosis and is important in triggering hormonal changes that occur after mating in female rodents [23,24,32]. Both the pudendal and pelvic nerves are also essential for micturition as they coordinate normal filling and voiding reflexes of the bladder and maintain continence [17–19]. The pelvic nerve regulates bladder contractions and the pudendal motor nerve controls external urethral sphincter activity. Recent studies have suggested that the pudendal sensory nerve can modulate micturition, possibly through a positive feedback loop by regulating the excitatory drive of the parasympathetic neurons [11,16]. Stimulation of the pudendal sensory nerve also increases vaginal blood flow (author’s unpublished observations). Therefore, there is a significant interaction between the afferent input and efferent output of the pudendal and pelvic nerves which may involve activation of common spinal circuits.
The afferent inputs from both the pudendal sensory and pelvic nerves enter the dorsal horn at L5-S1 segments. The pudendal nerve afferents course through and terminate in the superficial and medial dorsal horn and dorsal gray commissure (DGC) [46]. The pelvic nerve afferent fibers are located primarily in the superficial and lateral dorsal horn and project to the lateral gray [48,49]. In order to further understand the overlapping spinal networks that are activated in association with the coordination of the somatomotor and parasympathetic systems, the present study examined the distribution of spinal interneurons activated in two groups of animals that either received pudendal sensory or pelvic nerve stimulation.
Sensory transmission in the spinal cord is thought to be mediated primarily via glutamate, and localization of the vesicular glutamate transporters (VGlut) can be utilized to identify neurons that use glutamate as a neurotransmitter. VGlut2 and VGlut1 immunoreactivity are widely distributed in the central nervous system and are found in some overlapping regions [34,53,63]. Neurons synthesizing VGlut2 have been identified in the ventral medulla, a region that projects to the lumbosacral spinal cord and is involved in regulating pelvic function [42,60]. VGlut2 also innervates the parasympathetic preganglionic neurons of the lumbosacral spinal cord [38]. In contrast, the distribution of VGlut3 has been less well studied, and existing data suggests that VGlut3 exhibits a more restricted expression pattern compared to VGlut1 and VGlut2 [50,51]. VGlut3 has been shown in some cholinergic and serotonergic neurons in the brain and pelvic function is modulated by serotonergic and cholinergic supraspinal and spinal pathways [27,28,43]. Thus, we decided to examine the presence of VGlut2 and VGlut3 immunoreactivity in relationship to spinal neurons activated with nerve stimulation. In addition, substance P neurons containing neurokinin I receptors mediate nociceptive transmission in the dorsal horn and the preganglionic neurons and motoneurons mediating pelvic responses are cholinergic. Therefore, to provide information concerning the possible spinal neurotransmitters and mechanisms involved in relaying afferent input from the pudendal and pelvic nerves, we examined the distribution of c-fos with neurons containing glutamate transporters, choline acetyltransferase and neurokinin I receptors.
2. Results
2.1 Pudendal sensory nerve stimulation
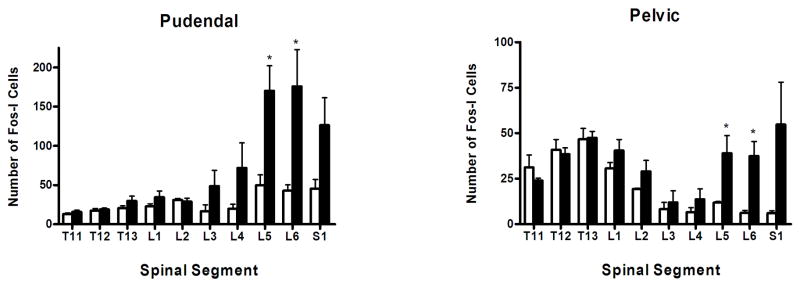
Following stimulation of the pudendal nerve a significant increase in fos-immunoreactive (fos-I) nuclei was observed in L5-S1 in the ipsilateral dorsal horn and bilaterally in the lateral, intermediate and medial gray (figures 1A, 1B and 2, table 1). The majority of fos-I nuclei were located in the superficial medial region of the dorsal horn (lamina I, II and III) and in the medial gray in the DGC and lamina X. In the intermediate gray fos-I nuclei were located in laminae V and VI and in the lateral gray fos-I nuclei were found in and around the sacral parasympathetic nucleus, where the parasympathetic preganglionic neurons are located (figure 1). A few fos-I nuclei were found in the dorso-medial ventral horn. Motoneurons did not exhibit any c-fos labeling. A stimulation induced increase in fos-immunoreactivity was also observed in L3-L4 segments bilaterally in the medial gray (table 1). A small stimulation induced increase in fos-immunoreactivity was also observed in the ipsilateral intermediate gray of L3-L4 and medial gray of T11-L2 (table 1).
Figure 1.
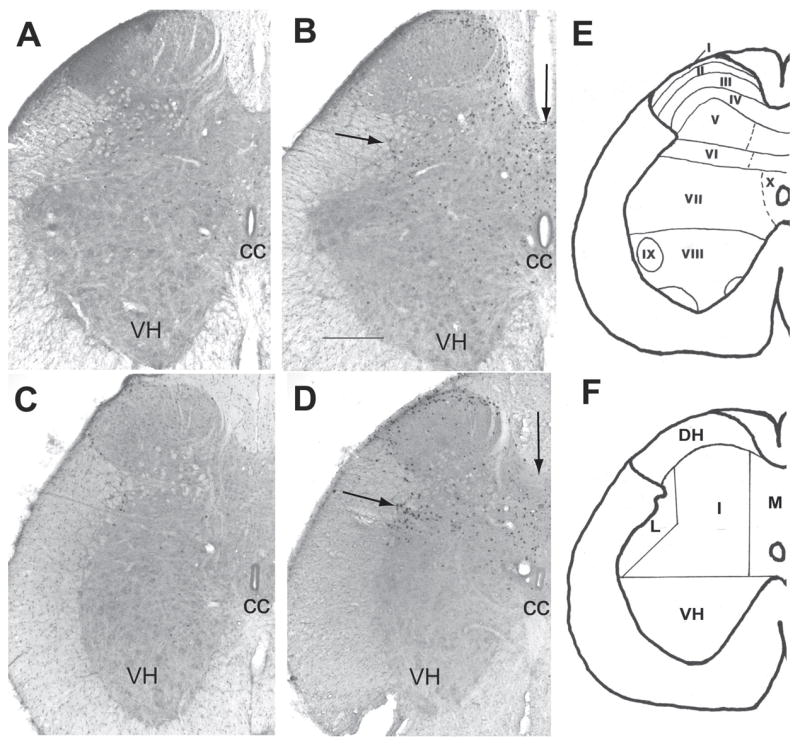
Photomicrographs of coronal sections of L5/6 spinal cord showing the distribution of fos-I nuclei [A–D]. [A] L5 pudendal nerve surgical control [B] L5 pudendal nerve stimulated [C] L6 pelvic nerve surgical control and [D] L6 pelvic nerve stimulated rat. Arrows in B and D show the location of the parasympathetic preganglionic neurons and the dorsal gray commissure. CC – central canal, VH – ventral horn. Scale bar = 500μm. [E] Spinal laminae I–X. [F] Regions counted. DH - dorsal horn, I -intermediate gray, L - lateral gray, M - medial gray and VH – ventral horn. Note the distribution pattern of fos-immunoreactivity in the dorsal horn and medial and lateral gray.
Figure 2.
Histograms showing the total number of fos-I nuclei in each spinal segment of surgical control and pudendal or pelvic nerve stimulated groups. Data represent mean ± SE (n = 4). Open bar - control group; filled bars – stimulated group. * represents a significant difference p< 0.05 from the surgical control group.
Table 1.
The number of fos-immunoreactive nuclei in the spinal cord after pudendal nerve stimulation.
| REGION | L5-S1 | L3-L4 | T13-L2 | T11-T12 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Stimulated | Control | Stimulated | Control | Stimulated | Control | Stimulated | ||
| DORSAL HORN | I | 21.7 ± 2.3 | 58.3 ± 9.2** | 7.8 ± 1.2 | 23.3 ± 7.7 | 10.7 ± 0.5 | 12.8 ± 1.1 | 6.1 ± 0.7 | 6.3 ± 0.6 |
| C | 11.4 ± 1.9 | 12.3 ± 1.9 | 4.6 ± 1.3 | 7.2 ± 1.7 | 5.9 ± 0.4 | 6.9 ± 0.8 | 3.5 ± 0.4 | 3.1 ± 0.5 | |
| LATERAL | I | 1.5 ± 0.3 | 6.6 ± 1.0*** | 0.4 ± 0.2 | 2.9 ± 0.8* | 1.3 ± 0.5 | 1.6 ± 0.3 | 1.1 ± 0.2 | 1.6 ± 0.2 |
| C | 0.8 ± 0.3 | 5.0 ± 0.7*** | 0.3 ± 0.1 | 1.9 ± 0.9 | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.2 | |
| INTERMEDIATE | I | 1.4 ± 0.3 | 6.9 ± 1.2** | 0.5 ± 0.1 | 2.7 ± 0.9* | 1.7 ± 0.3 | 2.7 ± 0.5 | 1.0 ± 0.2 | 1.6 ± 0.2* |
| C | 1.2 ± 0.4 | 6.1 ± 1.0*** | 0.3 ± 0.1 | 2.2 ± 0.9 | 1.2 ± 0.2 | 1.7 ± 0.4 | 0.7 ± 0.1 | 1.3 ± 0.2* | |
| MEDIAL | I | 3.6 ± 0.8 | 29.3 ± 4.9** | 2.1 ± 0.8 | 10.7 ± 3.0* | 1.3 ± 0.3 | 2.6 ± 0.4* | 0.9 ± 0.2 | 1.5 ± 0.1* |
| C | 2.3 ± 0.6 | 22.4 ± 3.6*** | 1.6 ± 0.6 | 8.2 ± 2.2* | 1.0 ± 0.3 | 2.6 ± 0.6* | 0.8 ± 0.2 | 0.9 ± 0.1* | |
| VENTRAL HORN | I | 0.2 ± 0.1 | 1.4 ± 0.4* | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| C | 0.2 ± 0.1 | 1.6 ± 0.3** | 1.0 ± 0.1 | 0.6 ± 0.2* | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 | |
Values are mean ± S.E., N = 6–12 per grouped segments (N = 4 animals per group),* denotes statistically significant differences between the control and stimulated group for each area and segments
p<0.05,
p<0.001,
p<0.0001; unpaired t-test. Abbreviations :- I- ipsilateral, C-contralateral.
2.2 Pelvic nerve stimulation
After stimulation of the pelvic nerve fos-I nuclei were seen in L5-S1 segments (figure 1C,1D and 2, table 2). A significant increase was found in the ipsilateral dorsal horn and lateral and intermediate gray. Pelvic nerve stimulation also activated neurons bilaterally in the medial gray. The majority of fos-I nuclei were located throughout the superficial lateral dorsal horn in lamina I and II (figure 1D). In the lateral gray, fos-I nuclei were located in the parasympathetic preganglionic nucleus and in lamina V and VI of the intermediate gray (figure 1D). In the medial gray fos-immunoreactivity was found primarily dorsal and ventral to the central canal in the DGC and lamina X. Pelvic nerve stimulation did not increase the number of fos-I nuclei in T13-L3 (figure 2, table 2).
Table 2.
The number of fos-immunoreactive nuclei in the spinal cord after pelvic nerve stimulation.
| REGION | L5-S1 | L3-L4 | T13-L2 | T11-T12 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Stimulated | Control | Stimulated | Control | Stimulated | Control | Stimulated | ||
| DORSAL HORN | I | 5.5 ± 0.7 | 26.8 ± 8.0* | 4.2 ± 1.5 | 5.3 ± 1.9 | 22.3 ± 2.4 | 26.0 ± 1.9 | 16.9 ± 3.4 | 16.9 ± 2.5 |
| C | 1.7 ± 0.3 | 4.3 ± 1.2 | 1.0 ± 0.3 | 1.5 ± 0.5 | 5.0 ± 0.6 | 5.1 ± 0.6 | 3.6 ± 0.4 | 3.4 ± 0.4 | |
| LATERAL | I | 0.4 ± 0.2 | 9.9 ± 2.4* | 0.1 ± 0.1 | 0.4 ± 0.2 | 1.2 ± 0.2 | 1.9 ± 0.3 | 2.4 ± 0.4 | 2.2 ± 0.2 |
| C | 0.1 ± 0.0 | 1.6 ± 0.4* | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 1.0 | 1.1 ± 0.2 | 1.3 ± 0.1 | |
| INTERMEDIATE | I | 0.1 ± 0.1 | 3.7 ± 0.9** | 0.4 ± 0.1 | 0.7 ± 0.2 | 2.8 ± 0.4 | 2.8 ± 0.5 | 3.9 ± 0.5 | 3.5 ± 0.2 |
| C | 0.2 ± 0.1 | 1.6 ± 0.5* | 0.2 ± 0.1 | 0.3 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.1 | |
| MEDIAL | I | 0.6 ± 0.1 | 9.8 ± 2.2* | 0.8 ± 0.1 | 2.2 ± 0.5* | 1.9 ± 0.3 | 2.2 ± 0.3 | 2.8 ± 0.3 | 2.1 ± 0.1 |
| C | 0.3 ± 1.0 | 8.3 ± 1.8** | 0.6 ± 0.2 | 1.4 ± 0.5 | 1.3 ± 0.3 | 1.6 ± 0.2 | 2.0 ± 0.2 | 1.6 ± 0.2 | |
| VENTRAL HORN | I | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| C | 0.0 ± 0.0 | 0.2 ± 0.1* | 0.0 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 | |
Values are mean ± S.E., N = 6–12 per grouped segments (N = 4 animals per group), * denotes statistically significant differences between the control and stimulated group for each area and segments
p<0.05,
p<0.001; unpaired t-test. Abbreviations :- I- ipsilateral, C- contralateral.
2.3 Overlapping spinal areas activated with pudendal sensory and pelvic nerve stimulation
A comparison of the spinal areas activated with pudendal sensory and pelvic nerve stimulation was made from matching L5-S1segments, where a significant increase in fos-I nuclei was found (see figure 1B, 1D and tables 1 and 2). The specific regions of the spinal gray matter that were activated by both nerves are represented in figure 3H. A small region of the medial and lateral dorsal horn (laminae I, II and the medial zone of lamina III) contained fos-I nuclei after stimulation of both nerves. Fos-I nuclei were seen after pudendal sensory and pelvic nerve stimulation in the DGC and dorsomedial part of lamina X. In addition, the lateral region of lamina V and a narrow band in the intermediate gray in lamina VI contained fos-I after stimulation of both nerves (figure 3). These areas represent the location of overlapping spinal interneurons that may coordinate or balance the autonomic/somatic regulation of sexual and voiding reflexes.
Figure 3.
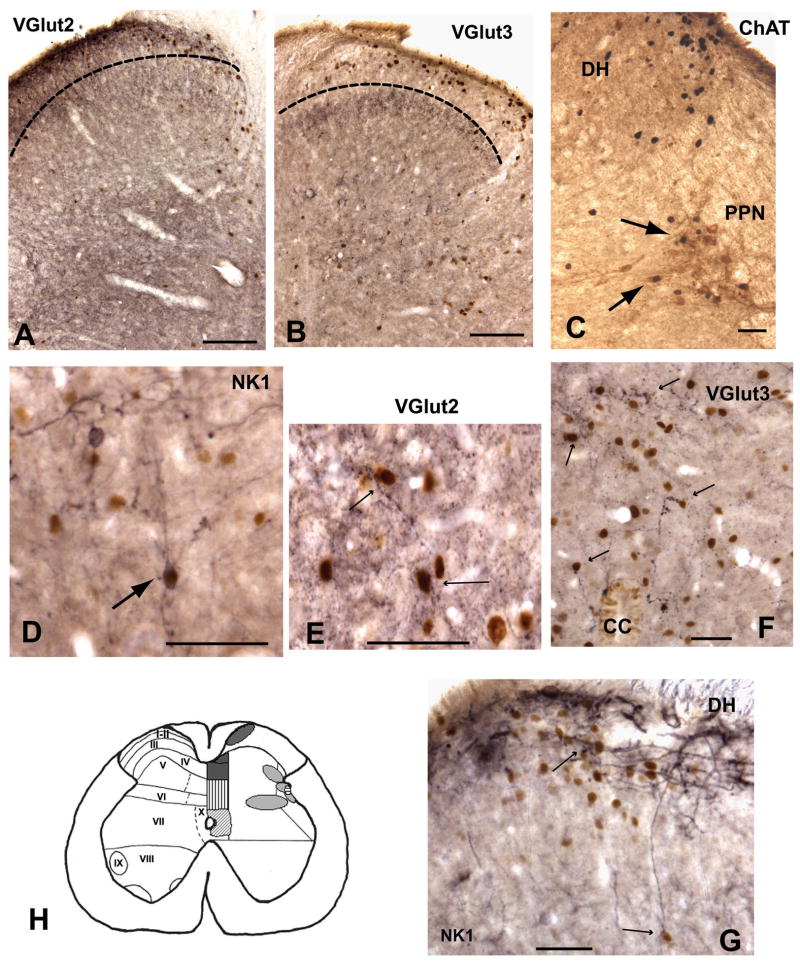
Photomicrographs illustrating the colocalization of fos-I nuclei with VGlut2 (A, E), VGlut3 (B, F), NKI (D, G) and ChAT (C). [A and B] Show the dorsal horn, the dotted line represents the approximate border of lamina II and lamina III. VGlut2 immunoreactivity is more prominent in the superficial laminae where it is co-distributed with fos-I nuclei. VGlut3 immunoreactivity is predominate in laminae III and not associated with fos-I nuclei in the superficial dorsal horn. [C] ChAT and fos-I nuclei in the dorsal horn and lateral gray. Arrows shows examples of cells colocalized with c-fos and ChAT in the PPN. [D and G] Examples of NKI activated cells in the dorsal horn (arrows). [E] VGlut2 immunoreactive dendrites and boutons adjacent to fos-I nuclei in the lateral gray (arrows). [F] VGlut3 immunoreactive dendrites and boutons associated with fos-I nuclei in the dorsal horn (arrows). Abbreviations:- DH - dorsal horn, CC -central canal, PPN - parasympathetic preganglionic nucleus. Scale bars = 250μm for A and B; 100μm for C-G. [H] Illustrates a summary diagram showing areas that resulted in increased fos-immunoreactivity after both pudendal sensory and pelvic nerve stimulation. The relationship of these areas with NKI, VGlut2 and VGlut3 is also shown. Left side shows the spinal laminae. Right side shows shaded areas in which fos-immunoreactivity was significantly increased with both pudendal and pelvic nerve stimulation. Specific shading subcategorizes areas showing significant overlap of fos-I nuclei with NKI, VGlut2 or VGlut3. Dark grey = NKI+VGlut2; vertical lines = VGlut3; diagonal lines = NKI; horizontal lines = VGlut2; and light shading = neither NKI, VGlut2 nor VGlut3.
2.4 Double immunocytochemistry
Examination of double labeling of fos-I nuclei and putative preganglionic neurons (ChAT positive), and overlap of fos-I nuclei with NKI receptors and VGlut2 and VGlut3 transporters was examined in L5-S1, where most of the activated neurons were located. Stimulation of the pelvic nerve resulted in an increase in the number of ChAT and fos-immunoreactive double labeled neurons in the parasympathetic preganglionic nucleus ipsilateral to the stimulated nerve (figure 3C). Around 2–5% of the total ChAT labeled cells were fos-positive in the control group and on the contralateral side in the nerve stimulated group, whereas, 10–15% of the ChAT neurons stained for c-fos in the ipsilateral parasympathetic preganglionic nucleus. In contrast, stimulation of the pudendal sensory nerve did not result in an increase in the percent of double labeled ChAT and c-fos positive neurons. In all groups 5–10% of the ChAT positive neurons in the parasympathetic preganglionic nucleus contained fos-immunoreactivity. Therefore, stimulation of the pelvic nerve, but not the pudendal nerve, activated parasympathetic preganglionic neurons.
The distribution of NKI receptors was similar to that previously described [62]; NKI receptor staining was primarily located in the superficial dorsal horn (laminae I and II) and appeared more prominent in the dorsomedial area (figure 3G). NKI receptors were also present in the lateral gray, DGC and the ventral horn. Numerous fos-I nuclei in the superficial dorsal horn were surrounded by NKI receptor immunoreactivity after pudendal or pelvic nerve stimulation (figure 3D,H and G). Other regions showing close overlap of NKI receptors and fos-I nuclei included the medial region of lamina IV and lamina X, and an occasional overlap was seen in the lateral gray after both pelvic and pudendal nerve stimulation.
As previously reported, [2,34,53] VGlut2 labeling was found to be distributed throughout the spinal gray matter and was particularly abundant in the superficial dorsal horn (laminae I and II; figure 3A) and in lamina IV. Therefore, some overlap of labeling of fos-I nuclei and VGlut2 was found in all regions after pudendal and pelvic nerve stimulation, but this overlap was most prominent in the dorsal horn and DGC (figure 3H). VGlut2 was also seen to be co-distributed with fos-I nuclei in the lateral gray (figure 3E).
The distribution of VGlut3 was more specific compared to VGlut2 [34]. VGlut3 labeling was primarily found in the deeper layers of the dorsal horn (laminae III/IV) which was ventral to the majority of fos-I nuclei (figure 3B). VGlut3 labeling was also present in the DGC, lateral and intermediate gray as well as being abundant in ventral horn motoneurons, including the dorsomedial and dorsolateral nuclei. An overlap of fos-immunoreactivity and VGlut3 was found in the DGC and lamina X surrounding the central canal (figure 3F and H). However, while VGlut3 immunoreactivity was found closely associated with some fos-I nuclei in the lateral gray, the majority of VGlut3 staining was ventral to the fos-I nuclei.
3. Discussion
This study demonstrates that stimulation of the pudendal sensory nerve or pelvic nerve results in activation (mapped using c-fos) of spinal neurons in L5-S1 of the spinal cord. Localized common areas of activation in the medial dorsal horn, DGC, lateral and intermediate gray were found after pudendal sensory and pelvic nerve stimulation suggesting these regions regulate the spinal integration of somatomotor and visceral changes seen during sexual function and micturition. An overlap of fos-I nuclei and NKI receptors and VGlut2 and VGlut3 transporters were found in these regions.
Unilateral nerve stimulation resulted in the greatest cell activation ipsilateral to the stimulus. However, fos-positive neurons were found on both sides of the DGC after either pudendal or pelvic nerve stimulation and in the contralateral lateral and intermediate gray after pudendal nerve stimulation. The pattern of fos immunoreactivity in the dorsal horn, medial and lateral gray matched the distribution of afferent fibers and terminals seen with anterograde tracing of the pudendal sensory or pelvic nerves [46,48,49]. The pudendal nerve afferents enter the superficial dorsal horn and course primarily in the medial dorsal horn and terminate in the DGC, in the exact locations that c-fos positive nuclei were observed [46]. In addition, an increase in fos-I nuclei was observed in the intermediate gray (laminae V and VI) and dorsal to the parasympathetic preganglionic nucleus which suggests that afferents also either directly or indirectly activate cells in these regions. Stimulation of the pelvic nerve resulted in increased fos-immunoreactivity in the superficial laminae (I and II) and lateral dorsal horn, and in the medial gray and parasympathetic preganglionic nucleus. This pattern of cell activation closely matches the description of pelvic afferent fibers and terminals coursing through the lateral dorsal horn which project towards the parasympathetic preganglionic nucleus, as well as afferent fibers projecting through the medial dorsal horn that travel to the DGC [49].
The pudendal and pelvic afferents modulate the level of tonic inhibition and excitatory drive that is important in regulating and coordinating lower urinary tract function through GABA and glutamate containing neurons. The superficial dorsal horn contains neurons which modulate afferent information through projections to local spinal laminae, other spinal segments and the brain [20,54,62]. The laminae I and II fos-I neurons labeled in the present study could be involved in nociception or regulation of muscle sensitivity and non noxious inputs [37].
Neurons in the DGC and lamina X activated with pudendal or pelvic nerve stimulation may integrate and relay afferent signals to efferent neurons within multiple spinal segments and relay important sensory information to the brain. Neuroanatomical studies mapping spinal interneurons involved in pelvic organ function confirm that the DGC and lamina X contain neurons that transverse multiple spinal segments and project to the brain [26,35,36,39–41]. Stimulation of the pudendal nerve and pelvic viscera increases electrophysiological activity of DGC neurons and their dendritic fields [25,30]. The pudendal motor neurons receive information from sensory inputs through their extensive dendritic branches which project into the medial gray; SPN neurons receive inputs through the DGC and via direct afferent inputs [9,14,44,46,65].
A small proportion of parasympathetic preganglionic neurons (ChAT and fos positive) were activated in response to pelvic nerve stimulation. However, most of the fos-I nuclei activated in this region were located surrounding the ChAT containing neurons and probably represent interneurons that communicate with the preganglionic neurons and brain nuclei [36,48,49,52]. Neurons in the lateral gray project to the pontine micturition center, periaqueductal gray and hypothalamus – areas known to regulate sexual and voiding function [21,22]. Neurons in this lateral region also respond to both cutaneous and visceral stimulation of both the colon and vagina, suggesting that fos-I neurons identified in the present study may coordinate and balance function between the various pelvic organs.
The present study agrees with a previous study that showed stimulation of the pelvic nerve activated neurons in the dorsal horn, medial and lateral gray of L6 and S1 [7]. However, they also reported a stimulus related increase in L1-L4, which was not observed in our study. Stimulation of the pelvic and pudendal nerves in the anesthetized cat resulted in an increase of fos-I nuclei in the superficial dorsal horn and medial gray of S1–S3 [29]. Other studies in females, have reported similar but relatively larger activation patterns of fos-immunoreactivity after vaginocervical stimulation, stimulation of the urethrogenital reflex and noxious stimulation of the bladder [5,6,13,15,26,35,41,58].
Multiple neurotransmitters in the spinal cord have been postulated to mediate sexual and bladder reflexes including glutamate, acetylcholine, oxytocin, serotonin and various peptides [3,10,27,58,59,66,69,70]. The present study examined the relationship of VGlut2, VGlut3 transporters and NKI receptors with fos-I nuclei as both glutamate and NKI receptors have been shown to regulate pelvic reflexes [64,69]. Glutamate is the major excitatory neurotransmitter and recent studies have documented the distribution of glutamate transporters in the lumbosacral cord [2,34,38,51,57,63]. Studies have suggested that VGlut1 and VGlut2 expression is restricted to glutaminergic neurons; however, VGlut3 expression is found not only in glutaminergic neurons but also neurons that contain acetylcholine and serotonin [28]. A recent study indicated that VGlut2 (not VGlut1) axons, supplied the majority of glutamate innervation of the parasympathetic preganglionic neurons [38] and the present study describes the overlap of VGlut2 with fos-I nuclei in the region of the parasympathetic preganglionic neurons in the lateral gray. Modulation of excitatory drive via glutamate AMPA receptors may occur in the dorsal horn since VGlut2 particularly overlapped with fos-I nuclei in the superficial dorsal horn, where glutamate release is thought to act on AMPA receptors [20]. Both VGlut2 and VGlut3 transporters may mediate spinal reflex excitatory drive in the DGC as they were both found to be codistributed with fos-I neurons. Glutamate transporters are primarily located on dendritic processes, thus the present study examining c-fos nuclei, rather than labeling the cell body and processes, may have limited our ability to identify all the glutamate inputs to the activated neurons. However, as shown in figure 3, VGlut 2 and VGlut3 immunostaining was frequently found in close proximity to the fos-I nuclei in the dorsal horn and medial and lateral gray. The presence of overlapping NKI receptors and fos-immunoreactivity in the medial dorsal horn and DGC suggests a functional role of NKI receptors in the regulation of lumbosacral spinal inputs that make collateral projections to deeper layers of the dorsal horn and project to the brain, in addition to regulating nociception [12,61,62,64].
A number of studies have provided evidence for cross organ interactions between gastrointestinal, bladder and reproductive pelvic organs particularly examining the responses of different organs to inflammation of a single organ such as the bladder, uterine horn or colon [56,68]. In addition, coordination of muscle relaxation and contraction and vasodilatation and vasoconstriction, takes place during sexual behavior and voiding reflexes. The communication of sensory information with coordination of the appropriate autonomic and motor output takes place at multiple levels of the nervous system including the dorsal root ganglia, spinal cord and the brain [4,55,56,68]. The present study reports the spinal cord regions that may relay cross communication between the viscera and somatomotor systems in the lumbosacral spinal cord.
4. Materials and Methods
Sprague-Dawley female ovariectomized rats (280–320 g) were anesthetized with urethane (n=16, 1.2 g/kg i.p.) prepared for either pudendal sensory or pelvic nerve stimulation. The pudendal nerve was exposed via a dorsal approach and the pelvic nerve via a ventral approach [8,46]. Body temperature was maintained at ~38°C with a heating pad. The jugular vein was cannulated in order to deliver extra anesthetic if required. The left pudendal sensory or pelvic nerve was stimulated (50μA, 40 Hz, 1 sec on/off) for 30 min via bipolar silver hook electrodes. These stimulation parameters were chosen as they produce consistent changes in vaginal blood flow and the urethrogenital reflex. Control animals were surgically prepared exactly the same as the stimulated group; however the pudendal sensory and pelvic nerves were exposed but not stimulated. After 60 minutes rest, animals were perfused with 4% paraformaldehyde while under deep anesthesia. The spinal cord was removed and placed into a 30% sucrose solution. All experimental procedures involving animals were approved by the University of North Carolina Institutional Animal Care and Use Committee in accordance with the Association for Assessments and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines.
The dorsal roots and ganglia were identified and coronal sections (35 μm) of T10-S2 of the spinal cord were cut on a freezing microtome and placed into cryoprotectant solution until processed [67]. A series of 1 in 6 sections (~170μm apart) were processed for identification of c-fos immunoreactive (fos-I) nuclei using the avidin-biotin immunoperoxidase method. Sections were incubated with rabbit anti-c-fos (1:80,000; DC38, Calbiochem, USA (previously Oncogene Research Products), synthetic peptide of amino acid residues 4–17 of human c-fos) overnight. Sections were washed in phosphate buffered saline (PBS) then incubated in biotinylated goat anti-rabbit IgG (1:500; BA1000, Vector Laboratories, USA) for ~1hr. Sections were washed in PBS and incubated in avidin-biotin complex (ABC, 1:1,000; PK6100, Vector Laboratories, USA) for 1hr. Sections were then incubated in DAB (3′3-diaminobenzidine tetrahydrochloride) – hydrogen peroxidase substrate containing nickel for 10 min. Control tissue was incubated in dilute normal (pre-immune) rabbit serum (1:6,000 dilution, Vector Laboratories, USA) or processed as above without the primary or secondary antibodies. These controls showed complete absence of staining. Sections were counterstained with methyl green (1%) in order to visualize the cytoarchitecture.
For visualization of fos-I nuclei and neurotransmitters a separate series was stained for c-fos (see above, with or without nickel) and then incubated in either goat anti-choline acetyl transferase (ChAT, 1:1,000; AB144P, Chemicon, USA), rabbit anti-Neurokinin I receptor, (NKI, 1:1000, ABN33AP, Advanced Targeting Systems, USA), guinea pig anti-vesicular glutamate transporter 2 or 3 (VGlut2 (AB5907) or VGlut3 (AB5421), 1:300,000 or 1:30,000 respectively, Chemicon, USA) overnight. Subsequently sections were incubated in 1:500 dilutions of biotinylated anti-goat IgG (ChAT, BA5000), biotinylated anti-rabbit IgG (NKI, BA1000), or biotinylated anti-guinea pig IgG (VGlut2 or VGlut3, BA7000) and visualized using the ABC method without nickel for ChAT, and with nickel for VGlut2, VGlut3 and NKI. Sections were also incubated for single immunostaining of each antibody. Controls for double staining included omission of one or both of each of the primary and secondary antibodies.
The spinal cord was delineated into five regions; dorsal horn (laminae I–III); intermediate gray (laminae IV, V, VI, VII); lateral gray (IML, lateral spinal nucleus and lateral gray of laminae IV, V, VI and VII); medial gray (lamina X, intermediomedial nucleus and laminae IV and V); and ventral horn (laminae VII, VIII, IX) (figure 1E and F) [41]. The number and location of fos-I nuclei in each region (dorsal horn, lateral gray, intermediate gray and ventral horn) were counted from T11-S2 for each animal (~every 170 μm, this strategy gave around 6–10 sections per segment). These data were then averaged according to segment. The data were examined by region and then grouped into T11–T12, T13-L2, L3-L4, and L5-S1. Comparisons between the surgical controls and nerve stimulated groups were done using one-way ANOVA followed by Scheffe’s post-hoc tests using SPSS 13.0 for Windows and unpaired t-test (Excel 6.0). Differences were considered significant when P < 0.05. Photomicrographs were generated using a Retiga 2000R camera attached to a Leica microscope. Images were captured using Adobe Photoshop 7.0. Alterations to images were limited to enhancement of brightness/contrast.
Acknowledgments
The authors would like to thank Dr Rong Cai and Karla Gravitt for their technical help.
The work was supported in part by NIH grant HDR01-30149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler NT, Davis PG, Komisaruk BR. Variation in the size and sensitivity of a genital sensory field in relation to the estrous cycle in rats. Horm Behav. 1977;9:334–44. doi: 10.1016/0018-506x(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–80. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- 3.Bancila M, Verge D, Rampin O, Backstrom JR, Sanders-Bush E, McKenna KE, Marson L, Calas A, Giuliano F. 5-Hydroxytryptamine2C receptors on spinal neurons controlling penile erection in the rat. Neuroscience. 1999;92:1523–37. doi: 10.1016/s0306-4522(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 4.Berkley KJ, Hubscher CH, Wall PD. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993;69:545–56. doi: 10.1152/jn.1993.69.2.545. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, de Groat WC. The effect of glutamate antagonists on c-fos expression induced in spinal neurons by irritation of the lower urinary tract. Brain Res. 1992;580:115–20. doi: 10.1016/0006-8993(92)90934-2. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–33. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 7.Birder LA, Roppolo JR, Iadarola MJ, de Groat WC. Electrical stimulation of visceral afferent pathways in the pelvic nerve increases c-fos in the rat lumbosacral spinal cord. Neurosci Lett. 1991;129:193–6. doi: 10.1016/0304-3940(91)90459-7. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RR, De Feo VJ. Role of the pelvic nerve vs. the abdominal sympathetic nerves in the reproductive function of the female rat. Endocrinology. 1965;77:1014–22. doi: 10.1210/endo-77-6-1014. [DOI] [PubMed] [Google Scholar]
- 9.Cayzergues L, Yaici el D, Tabard SB, Jestin A, Blanchard P, Giuliano F, Bensadoun H, Jardin A, Benoit G, Droupy S. Morphological study of the spinal motoneurons controlling the urethral sphincter of female rats: role of androgens in a menopausal model. J Urol. 2005;173:1022–6. doi: 10.1097/01.ju.0000146269.43658.d3. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292:F1044–53. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HY, Cheng CL, Chen JJ, Peng CW, de Groat WC. Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats. J Neurosci Methods. 2006;150:80–9. doi: 10.1016/j.jneumeth.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman V, Buritova J, Honore P, Besson JM. Physiological contributions of neurokinin 1 receptor activation, and interactions with NMDA receptors, to inflammatory-evoked spinal c-Fos expression. J Neurophysiol. 1996;76:1817–27. doi: 10.1152/jn.1996.76.3.1817. [DOI] [PubMed] [Google Scholar]
- 13.Chinapen S, Swann JM, Steinman JL, Komisaruk BR. Expression of c-fos protein in lumbosacral spinal cord in response to vaginocervical stimulation in rats. Neurosci Lett. 1992;145:93–6. doi: 10.1016/0304-3940(92)90211-o. [DOI] [PubMed] [Google Scholar]
- 14.Collins WF, 3rd, Erichsen JT, Rose RD. Pudendal motor and premotor neurons in the male rat: a WGA transneuronal study. J Comp Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- 15.Cruz F, Avelino A, Lima D, Coimbra A. Activation of the c-fos proto-oncogene in the spinal cord following noxious stimulation of the urinary bladder. Somatosens Mot Res. 1994;11:319–25. doi: 10.3109/08990229409028876. [DOI] [PubMed] [Google Scholar]
- 16.Cruz Y, Downie JW. Abdominal muscle activity during voiding in female rats with normal or irritated bladder. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1436–45. doi: 10.1152/ajpregu.00556.2005. [DOI] [PubMed] [Google Scholar]
- 17.de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20:383–401. [PubMed] [Google Scholar]
- 18.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–60. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 19.de Groat WC, Steers WD. Central Regulation of Autonomic Function. Oxford University Press; 1990. Autonomic regulation of the urinary bladder and sexual organs; pp. 310–333. [Google Scholar]
- 20.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28:633–8. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 21.Ding YQ, Wang D, Nie H, Guan ZL, Lu BZ, Li JS. Direct projections from the periaqueductal gray to pontine micturition center neurons projecting to the lumbosacral cord segments: an electron microscopic study in the rat. Neurosci Lett. 1998;242:97–100. doi: 10.1016/s0304-3940(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 22.Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ. Direct projections from the lumbosacral spinal cord to Barrington’s nucleus in the rat: a special reference to micturition reflex. J Comp Neurol. 1997;389:149–60. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Erskine MS. Prolactin release after mating and genitosensory stimulation in females. Endocr Rev. 1995;16:508–28. doi: 10.1210/edrv-16-4-508. [DOI] [PubMed] [Google Scholar]
- 24.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Fedirchuk B, Song L, Downie JW, Shefchyk SJ. Spinal distribution of extracellular field potentials generated by electrical stimulation of pudendal and perineal afferents in the cat. Exp Brain Res. 1992;89:517–20. doi: 10.1007/BF00229876. [DOI] [PubMed] [Google Scholar]
- 26.Ghanima A, Bennis M, Rampin O. c-Fos expression as endogenous marker of lumbosacral spinal neuron activity in response to vaginocervical-stimulation. Brain Res Brain Res Protoc. 2002;9:1–8. doi: 10.1016/s1385-299x(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 27.Gomez LE, Flores R, Cano P, Duran I, Cueva-Rolon R. Cholinergic modulation of the urethro genital reflex in spinal cord-transected rats. Pharmacol Biochem Behav. 2005;81:100–13. doi: 10.1016/j.pbb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill WM, Wang B, Hadziefendic S, Haxhiu MA. Identification of the spinal neural network involved in coordination of micturition in the male cat. Brain Res. 1998;796:150–60. doi: 10.1016/s0006-8993(98)00340-0. [DOI] [PubMed] [Google Scholar]
- 30.Honda CN. Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J Neurophysiol. 1985;53:1059–78. doi: 10.1152/jn.1985.53.4.1059. [DOI] [PubMed] [Google Scholar]
- 31.Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004;171:1357–61. doi: 10.1097/01.ju.0000109868.19569.d7. [DOI] [PubMed] [Google Scholar]
- 32.Kollar EJ. Reproduction in the female rat after pelvic nerve neurectomy. Anat Rec. 1953;115:641–58. doi: 10.1002/ar.1091150406. [DOI] [PubMed] [Google Scholar]
- 33.Kow LM, Pfaff DW. Effects of estrogen treatment on the size of receptive field and response threshold of pudendal nerve in the female rat. Neuroendocrinology. 1973;13:299–313. doi: 10.1159/000122214. [DOI] [PubMed] [Google Scholar]
- 34.Landry M, Bouali-Benazzouz R, El Mestikawy S, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468:380–94. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Erskine MS. Vaginocervical stimulation suppresses the expression of c-fos induced by mating in thoracic, lumbar and sacral segments of the female rat. Neuroscience. 1996;74:237–49. doi: 10.1016/0306-4522(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Erskine MS. Pseudorabies virus tracing of neural pathways between the uterine cervix and CNS: effects of survival time, estrogen treatment, rhizotomy, and pelvic nerve transection. J Comp Neurol. 2000;418:484–503. [PubMed] [Google Scholar]
- 37.Light AR, Perl ER. Unmyelinated afferent fibers are not only for pain anymore. J Comp Neurol. 2003;461:137–9. doi: 10.1002/cne.10691. [DOI] [PubMed] [Google Scholar]
- 38.Llewellyn-Smith IJ, Martin CL, Fenwick NM, Dicarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol. 2007;503:741–67. doi: 10.1002/cne.21414. [DOI] [PubMed] [Google Scholar]
- 39.Marson L. Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett. 1995;190:41–4. doi: 10.1016/0304-3940(95)11495-i. [DOI] [PubMed] [Google Scholar]
- 40.Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- 41.Marson L, Cai R, Makhanova N. Identification of spinal neurons involved in the urethrogenital reflex in the female rat. J Comp Neurol. 2003;462:355–70. doi: 10.1002/cne.10732. [DOI] [PubMed] [Google Scholar]
- 42.Marson L, McKenna KE. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Res. 1990;515:303–8. doi: 10.1016/0006-8993(90)90611-e. [DOI] [PubMed] [Google Scholar]
- 43.Marson L, McKenna KE. A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Exp Brain Res. 1992;88:313–20. doi: 10.1007/BF02259106. [DOI] [PubMed] [Google Scholar]
- 44.Mawe GM, Bresnahan JC, Beattie MS. A light and electron microscopic analysis of the sacral parasympathetic nucleus after labelling primary afferent and efferent elements with HRP. J Comp Neurol. 1986;250:33–57. doi: 10.1002/cne.902500104. [DOI] [PubMed] [Google Scholar]
- 45.McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. Am J Physiol. 1991;261:R1276–85. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- 46.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–49. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 47.Min K, Munarriz R, Kim NN, Choi S, O’Connell L, Goldstein I, Traish AM. Effects of ovariectomy and estrogen replacement on basal and pelvic nerve stimulated vaginal lubrication in an animal model. J Sex Marital Ther. 2003;29(Suppl 1):77–84. doi: 10.1080/713847131. [DOI] [PubMed] [Google Scholar]
- 48.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–40. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 49.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–45. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Wu SX, Fujiyama F, Okamoto K, Hioki H, Kaneko T. Independent inputs by VGLUT2- and VGLUT3-positive glutamatergic terminals onto rat sympathetic preganglionic neurons. Neuroreport. 2004;15:431–6. doi: 10.1097/00001756-200403010-00010. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–29. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- 52.Papka RE, McCurdy JR, Williams SJ, Mayer B, Marson L, Platt KB. Parasympathetic preganglionic neurons in the spinal cord involved in uterine innervation are cholinergic and nitric oxide-containing. Anat Rec. 1995;241:554–62. doi: 10.1002/ar.1092410413. [DOI] [PubMed] [Google Scholar]
- 53.Persson S, Boulland JL, Aspling M, Larsson M, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA, Broman J. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol. 2006;497:683–701. doi: 10.1002/cne.20987. [DOI] [PubMed] [Google Scholar]
- 54.Puder BA, Papka RE. Activation and circuitry of uterine-cervix-related neurons in the lumbosacral dorsal root ganglia and spinal cord at parturition. J Neurosci Res. 2005;82:875–89. doi: 10.1002/jnr.20690. [DOI] [PubMed] [Google Scholar]
- 55.Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport. 2004;15:467–71. doi: 10.1097/00001756-200403010-00017. [DOI] [PubMed] [Google Scholar]
- 56.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–78. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Ranson RN, Santer RM, Watson AH. Ageing reduces the number of vesicular glutamate transporter 2 containing immunoreactive inputs to identified rat pelvic motoneurons. Exp Gerontol. 2007;42:506–16. doi: 10.1016/j.exger.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol. 2005;288:F466–73. doi: 10.1152/ajprenal.00274.2004. [DOI] [PubMed] [Google Scholar]
- 59.Stafford SA, Tang K, Coote JH. Sympathetic genital responses induced by p-chloroamphetamine in anaesthetized female rats. Neuroscience. 2006;138:725–32. doi: 10.1016/j.neuroscience.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 60.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–20. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 61.Tao YX, Zhao ZQ. [Neurokinin-1 receptor mediated formalin-induced c-fos expression in the rat spinal cord] Sheng Li Xue Bao. 1998;50:361–6. [PubMed] [Google Scholar]
- 62.Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–9. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- 63.Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 64.Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–13. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 66.Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, Stoeckel ME, Benoit G, Giuliano F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–47. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 67.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–9. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 68.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1592–601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 69.Yoshiyama M, de Groat WC. Supraspinal and spinal alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132:1017–26. doi: 10.1016/j.neuroscience.2005.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshiyama M, Erickson KA, Erdman SL, De Groat WC. Effects of N-methyl-D-aspartate (dizocilpine) and alpha-amino-3-hydroxy-4-isoxazolepropionate ( LY215490) receptor antagonists on the voiding reflex induced by perineal stimulation in the neonatal rat. Neuroscience. 1999;90:1415–20. doi: 10.1016/s0306-4522(98)00545-4. [DOI] [PubMed] [Google Scholar]