Abstract
Worldwide, approximately 200 million people currently have type II diabetes mellitus (DM), a prevalence that has been predicted to increase to 366 million by 2030. Rates of cardiovascular disease (CVD) mortality and morbidity are particularly high in this population, representing a significant cost for health care systems. Type II DM patients generally carry a number of risk factors for CVD, including hyperglycemia, abnormal lipid profiles, alterations in inflammatory mediators and coagulation/thrombolytic parameters, as well as other ‘nontraditional’ risk factors, many of which may be closely associated with insulin resistance. Therefore, successful management of CVD associated with diabetes represents a major challenge to the clinicians. An effective way of tackling this problem is to detect the associated risk factors and to target treatment toward their improvement. Targeting hyperglycemia alone does not reduce the excess risk in diabetes, highlighting the need for aggressive treatment of other risk factors. Although the current use of statin therapy is effective at reducing low-density lipoprotein cholesterol, residual risk remains for other independent lipid and nonlipid factors. The peroxisome proliferator-activated receptor-γ appears to be closely involved in regulating risk markers at multiple levels. A relatively new class of therapeutic agents that activate peroxisome proliferator-activated receptor-γ, the thiazolidinedione insulin-sensitizing agents, is currently used to manage type II DM. These agents display a number of potential antiatherogenic properties, including effects on high-density lipoprotein cholesterol and triglycerides, as well as other beneficial nonlipid effects, such as regulating levels of mediators involved in inflammation and endothelial dysfunction. Research data suggest that simple strategies combining thiazolidinediones and statins could have complementary effects on CVD risk-factor profiles in diabetes, alongside the ability to control glycemia.
Keywords: Coronary artery disease, PPAR-γ, Therapy, Type II diabetes
Diabetes mellitus (DM) is a metabolic disorder principally characterized by elevated blood glucose levels and by microvascular and macrovascular complications that considerably increase the morbidity and mortality related to the disease (1,2). Type I DM (insulin-dependent diabetes mellitus, IDDM) is characterized by a near-total reliance on exogenous insulin for survival, and long-standing type I DM patients are susceptible to microvascular complications, including nephropathy, retinopathy and neuropathy, specific to diabetes and to nonspecific macrovascular disease (coronary artery disease [CAD] and peripheral vascular disease, [Table 1]). Mortality in type I DM has increased four- to sevenfold over the matched nondiabetic population, and nephropathy and CAD are the main causes of death (3–5). However, type II DM (noninsulin-dependent diabetes mellitus, NIDDM) is characterized by relative insulin deficiency and/or insulin resistance and is becoming more common than type I, usually occurring in middle age, most commonly in the obese. The reason for this is attributed, in part, to an aging population and the increasing prevalence of obesity and sedentary lifestyles (3).
TABLE 1.
Causes of mortality in type I and type II diabetes mellitus (DM)
| Type I DM (%) | Type II DM (%) | |
|---|---|---|
| Cardiovascular disease | 15 | 58 |
| Cerebrovascular disease | 3 | 12 |
| Nephropathy | 55 | 3 |
| Diabetic ‘coma’ | 4 | 1 |
| Malignancy | 0 | 11 |
| Infections | 10 | 4 |
| Others | 13 | 11 |
Specific microvascular complications in type II DM are less common than in type I DM, in which the onset is earlier and exposure to the disease is generally longer. However, retinopathy (especially maculopathy rather than proliferative changes), nephropathy and neuropathy occur (Table 1). Type II DM carries a high risk of large-vessel atherosclerosis, where the lining of the artery wall becomes enlarged, as cells from the blood, along with lipids, accumulate, ultimately weakening the wall and precipitating a rupture. This condition affects many individuals and is commonly associated with hypertension, hyperlipidemia and obesity (5–9). Myocardial infarction (MI) is also common and accounts for 60% of deaths. Overall mortality of type II DM has increased two- to threefold and life expectancy is reduced by five to 10 years (3).
Atherosclerotic CAD and other forms of cardiovascular disease (CVD) are the major causes of mortality in type II DM (1,2), and are major contributors to morbidity and depreciation in quality of life (3,4). Risks of incidence from CAD or fatal CAD are two- to fourfold higher in people with DM than in those without (5–9). Furthermore, long-term prognosis after a coronary event is significantly worse among people with DM than those without (10). Patients with type II DM (but without previous MI) have as high a risk of MI as nondiabetic patients with previous MI (5). Accordingly, the National Cholesterol Education Program (NCEP) guidelines classify DM as a CAD “risk equivalent” – a disorder that carries an absolute, 10-year risk for developing new major coronary events equal to that of nondiabetic persons with established CAD (ie, less then 20%) (11). Thus, DM is considered an important cause of CVD and, from the perspective of cardiovascular medicine, may even be considered as a CVD in itself (12). However, the core metabolic defect in DM (ie, hyperglycemia) does not by itself raise the risk to the level of a CAD risk equivalent – it is a constellation of metabolic risk factors that combine with hyperglycemia to impart a high risk (11). The present review describes the various CVD risk markers presenting in people with type II DM and indicates how current and potential future strategies aim to reduce this CVD risk.
INTERRELATION OF METABOLIC ABNORMALITIES AND CARDIOVASCULAR COMPLICATIONS ASSOCIATED WITH TYPE II DM
The major underlying defect of type II DM is insulin resistance and progressive deterioration of β-cell functions (1). A diversity of causes, including aging, genetic defects, environmental factors and obesity, can trigger the development of insulin resistance. Once insulin resistance develops in several tissues, insulin-stimulated glucose disposal is decreased and adipocytes release many free fatty acids (FFAs). Furthermore, increased FFAs inhibit the insulin action on liver, resulting in increased gluconeogenesis in the hyperglycemic state.
Coronary artery atherosclerosis in diabetic subjects is more diffuse and severe than in nondiabetic subjects. Acute MI in diabetes carries twice the mortality of that in the general population and contributing factors may include coexistent diabetic cardiomyopathy, autonomic neuropathy, and adverse cardiac and metabolic effects of increased nonesterified fatty acid levels (6–10). Acute MI in these subjects is usually managed by tight control of blood glucose and potassium levels and prompt treatment of cardiac failure. The symptoms of angina can be masked in diabetic patients by autonomic neuropathy (11–21).
DM patients often present a number of discrete CVD risk factors (Table 2) that are closely associated with the presence of insulin resistance, a possible common etiological factor, although without clear connection (11,13). The combination of multiple CVD risk factors and insulin resistance is collectively known as the ‘metabolic syndrome’ as defined by the World Health Organization and the NCEP Adult Treatment Panel (ATP) III (11,13) (Table 2). Type II DM patients with insulin resistance have a proatherogenic cardiovascular risk profile which includes impaired glucose regulation, abdominal obesity, hypertension, atherogenic dyslipidemia (characterized by elevated levels of triglycerides [TGs] and low levels of high-density lipoprotein cholesterol [HDL-C]), microalbuminuria, and specific proinflammatory and prothrombotic abnormalities of endothelial cell and vascular functions (11,13–15). In those with normal glucose tolerance, the presence of the metabolic syndrome also predicts a high risk for developing type II DM (13,16,17).
TABLE 2.
Coronary artery disease risk factors associated with type II diabetes mellitus
| Risk factors | Reference |
|---|---|
| Hyperglycemia | 11, 31, 40 |
| Hyperinsulinemia | 11, 12 |
| Obesity | 13–15 |
| Dyslipidemia | 22–26 |
| Decreased high-density lipoprotein cholesterol | |
| Small, dense low-density lipoprotein particle size | |
| Increased triglycerides | |
| Hypertension | 17, 18 |
| Microalbuminuria | 40 |
| High white blood cell count | 21, 27, 40 |
| Vascular inflammation markers | 30–32, 36–39 |
| Increased C-reactive protein | |
| Increased monocyte chemotactic protein-1 | |
| Increased proinflammatory cytokines | |
| Coagulation and thrombotic markers | 11, 13–15, 76–78 |
| Increased mean platelet volume | |
| Decreased antioxidant status | |
| Increased von Willebrand factor | |
| Decreased antithrombin III | |
| Increased plasminogen activator inhibitor-1 | |
| Increased fibrinogen | |
| Increased matrix metalloproteinase levels | |
| Endothelial dysfunction | 30–32, 41, 42 |
| Decreased vascular reactivity | |
| Increased degradation of nitric oxide | |
| Reduced release of prostacyclin | |
| Impaired flow-mediated dilation |
Although each component of the metabolic syndrome brings an individual increased CVD risk, the effect is enhanced when in combination. Therefore, the metabolic syndrome is associated with a threefold increase in the risk of CAD and stroke and a threefold increase in the likelihood of mortality from CAD (18,19). Furthermore, the risk of CVD (and also DM) increases as the number of components of the metabolic syndrome increases (16).
The prevalence rate of the metabolic syndrome is high in many western countries, with 25% to 35% of the general population affected. Based on 2002 census data of adults in the United States, approximately 47 million residents have the metabolic syndrome and the age-adjusted prevalence of the metabolic syndrome is 24%, increasing with age from 7% in the Third National Health and Nutrition Examination Survey (NHANES III) participants aged 20 to 29 years to 44% in those aged 60 to 69 years (20). The association with DM was highlighted in a recent analysis from NHANES III in which approximately 85% of people with diabetes were classified as having the metabolic syndrome compared with only 12% of those with normal fasting glucose (21).
Abnormal lipid profiles
Dyslipidemia is an established risk factor for CAD in patients with type II DM, as well as in nondiabetic patients, and is likely to play a leading role in the increased CVD risk associated with diabetes (11,22,23). The dyslipidemia associated with type II DM is typically more complex than simple elevation of systemic low-density lipoprotein cholesterol (LDL-C) levels. In fact, the LDL-C levels seen in diabetic populations may not be significantly different from the values seen in nondiabetic populations. The high atherogenicity associated with diabetic dyslipidemia is probably related to the characteristic finding of low plasma concentrations of HDL-C, elevated levels of apolipoprotein B and elevated TG levels, as well as to abnormalities in lipoprotein particle size and subclass distribution (24,25). Among the wide range of lipoprotein subclasses that have been described, disproportionate amounts of small, dense LDL particles and small HDL particles are thought to constitute a particularly atherogenic profile due to a high susceptibility to oxidation (26).
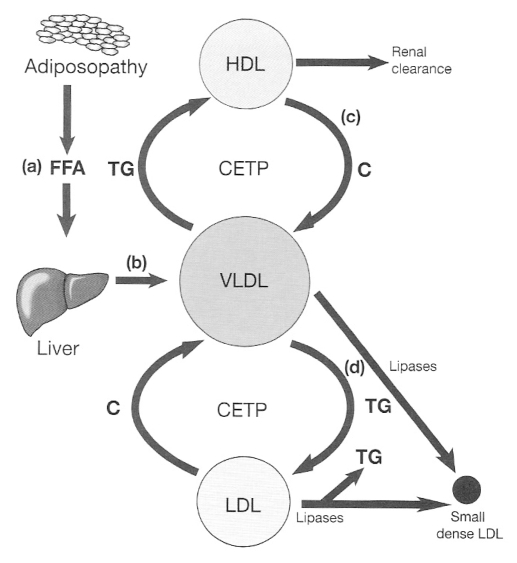
Several mechanisms may account for the atherogenic lipid abnormalities in diabetic patients. Dysfunctional adipose tissue or adiposopathy is thought to develop via the combination of excessive fat accumulation and genetic predisposition. Evidence suggests that this dysfunctional adipose tissue is less sensitive to insulin and has reduced hormone-sensitive lipase activity compared that of with normal adipose tissue. As a result, there is an increased breakdown of intracellular TG and increased release of FFAs into the circulation, leading to fatty infiltration in the liver, muscles and possibly pancreatic β-cells (Figure 1a). Ultimately, this contributes to, and may exacerbate, insulin resistance in the liver and muscle. After long-term exposure to FFA, the function of the pancreatic β-cells may also be compromised, leading to or contributing to increased predisposition to type II DM. Increased hepatic FFAs contribute to increased hepatic synthesis of TG, which in turn results in elevated concentrations of very LDL (VLDL) particles (Figure 1b). As a consequence, the characteristic hyper-triglyceridemia (and possible ‘fatty liver’) emerges, a common finding in patients with insulin resistance. Afterwards, various lipases contribute to remodelling of VLDL to small, dense LDL particles (Figure 1d). In addition, cholesteryl ester transfer protein (CETP) exchanges TG from VLDL to cholesterol found in HDL and LDL, leading to cholesterol-rich atherogenic VLDL particles. HDL particles that undergo these modifications are cleared more readily by the kidney, resulting in lower HDL-C levels (Figure 1c). The more TG-rich LDL particles undergo metabolism by lipases, again resulting in small, dense LDL particles that exhibit increased atherogenicity.
Figure 1.
The role of cholesteryl ester transfer protein (CETP) in the creation of an atherogenic lipid profile of hypertriglyceridemia, cholesterol (C)-rich very low-density lipoprotein (VLDL), low high-density lipoprotein (HDL)-C levels and small, dense LDL particles typical of the metabolic syndrome and type II diabetes mellitus (reproduced from reference 99). FFA Free fatty acid; TG Triglyceride
Hypertension
Hypertension in diabetic patients represents an important health problem because the combination of the two diseases is common. Hypertension affects over 30% of European diabetic patients and is twice as common as in the nondiabetic population (27). DM predisposes individuals to hypertension by promoting sodium retention, increasing vascular tone and by contributing to nephropathy. Hypertension in type II DM can be partly a consequence of insulin resistance and of hyperinsulinemia. Aortic stiffness as measured from aortic pulse wave velocity (PWV) has been shown to be a predictor of future cardiovascular events in patients with DM and hypertension (28,29). It is of note (30) that an increase in brachial-ankle PWV is associated with symptomatic cerebral infarction in patients with type II DM. Indeed, there are significant differences in the age-related increase in vascular stiffness in the elastic arteries of people without diabetes, compared with those in the arteries of patients with diabetes, and their blood vessels seem to age at an accelerated pace, starting at an earlier age and then reaching a functional plateau (31). Thus, PWV is thought to be useful as a marker relating to the severity of atherosclerosis and for predicting future atherosclerotic cardiovascular events in DM patients.
Endothelial dysfunction and the vascular wall in DM
Vascular endothelial dysfunction has been proposed to play a pivotal role in the development and progression of subclinical atherosclerosis. The properties of the impaired endothelium include reduced vasoactive capability, increased ability to support thrombosis, increased permeability and increased adhesion molecule expression (32,33). Such changes produce increased adhesion of leukocytes and platelets, increased responsiveness to vasoconstrictor agents (eg, angiotensin II, endothelin-1 and thrombin) and increased transmigration of leukocytes (33,34). Prostacyclin and nitric oxide (NO), produced by normal endothelium, inhibit platelet activation and relax vascular smooth muscle, promoting normal blood flow. People with DM have a reduced release of prostacyclin and NO (35) and the chronic impairment of endothelial NO synthase activity by this mechanism may partly explain the accelerated atherosclerosis in DM. Endothelial dysfunction can be detected early in the prediabetic state and the progression of endothelial dysfunction to atherosclerosis parallels that of insulin resistance to type II DM (32,33).
Plasma von Willebrand factor (vWF) (a marker of endothelial damage/dysfunction) levels are elevated in patients with type II DM, particularly in the presence of microalbuminuria and a history of CAD. This was found to be associated with markers of an increased oxidative stress and therefore reflect the severity of biochemical abnormalities, contributing to diabetic vascular disease (36). Increased levels of vWF was found to be independently and significantly associated with diabetes in a cohort of postinfarction patients, possibly indicative of endothelial damage being causal to the increased vascular events (37).
Impaired endothelial synthesis of NO is an important feature of atherothrombosis, estimated from endothelium-dependent flow-mediated dilation (FMD), and type II DM is independently associated with impaired FMD (38). Diabetic patients also have a decreased FMD and an increased arterial stiffness compared with age- and sex-matched nondiabetic people, and these functional changes correlate well with the structural changes of the arteries measured by intimal medial thickness (39).
The relationship between micro- or macroalbuminuria and CVD mortality may also be related to its association with endothelial dysfunction (40,41).
Inflammation and diabetes
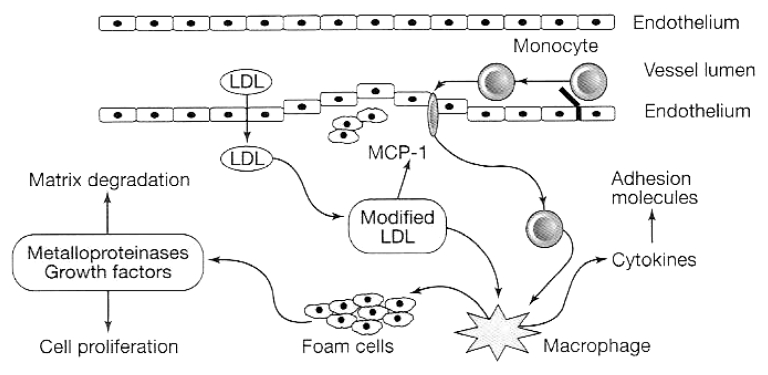
There is a close association between inflammation and endothelial dysfunction and there is increasing evidence that low-grade inflammation, perhaps reflecting a widespread activation of the innate immune system, is closely involved in the pathogenesis of type II DM dyslipidemia and atherosclerosis (42). The ongoing acute phase response (seen in insulin-resistant subjects and type II DM patients) is induced by cytokines, and is reflected in elevated circulating inflammatory markers, such as C-reactive protein (CRP), interleukin (IL)-1, IL-6, tumour necrosis factor-alpha (TNF-α), leptin, plasminogen activator inhibitor-1 (PAI-1), angiotensinogen and fibrinogen (described by Hsueh and Bruemmer [43] as a “proinflammatory milieu”). Such chronic inflammation of the endothelial cell and vascular environment impairs endothelium-dependent vasodilation, induces the expression of cell surface adhesion molecules by endothelial cells and increases cardiovascular risk (44–46) (Figure 2). In particular, CRP may play a significant role as it amplifies the inflammatory response by stimulating the production of TNF-α and IL-1 by tissue macrophages (46). Thus, CRP has been linked with CAD mortality and the development of DM (42,47,48). CRP also stimulates PAI-1, which inhibits fibrinolysis and also predicts CAD and DM, as well as contributing to the prothrombotic state in obesity (43,46).
Figure 2.
Inflammatory processes involved in the development of atherosclerotic lesions (reproduced from reference 99). LDL Low-density lipoprotein; MCP-1 Monocyte chemotactic protein-1
There is also evidence to suggest that there is an association between diabetes and cyclooxygenase-2-mediated inflammation (49). In the presence of proinflammatory stimuli, endothelial cells are activated by an increasing production and expression of soluble adhesion molecules, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin (50,51). Moreover, prospective studies (52,53) have showed that elevated levels of intercellular adhesion molecule-1 and E-selectin in diabetes significantly predict future risk of CVD. CD40 ligand is a trimeric, transmembrane protein of the TNF family and, together with its receptor, is an important contributor to the inflammatory processes that lead to atherosclerosis and thrombosis. Both CD40 and CD40 ligand have been showed to be present in human atheroma (54), and intensive multifactorial cardiovascular risk management reduces high levels of soluble CD40 ligand in diabetes, particularly in patients with overt CVD (55).
Abnormalities of extracellular matrix turnover
Circulating markers of extracellular matrix turnover, such as matrix metalloproteinases (MMPs) and their tissue inhibitors of metalloproteinases (TIMPs) are fundamental to the vascular changes of atheromatous vascular disease. TIMP-1 is an endogenous MMP inhibitor that may be involved in vascular matrix fibrosis (56), and has a role in left ventricular hypertrophy and diastolic dysfunction by reducing cardiac collagen type I turnover and increasing cardiac mass and stiffness (57). It is of note (58) that MMP-9 and TIMP-1 levels are significantly raised in DM, and increased central and peripheral artery stiffness is also present in this condition (59). Reductions in TIMP-1 levels have been observed with improvement in metabolic control, and this raises the possibility of TIMP-1 as a marker of vascular composition in diabetes (60), a possible target for intervention.
Hyperglycemia and oxidative stress
Hyperglycemia may induce protein glycation, oxidation, glycoxidation and lipoxidation, and may mediate vascular damage in DM (61,62). Connective tissue proteins, such as collagen, may be affected by elevated glucose levels and it has been observed that in DM individuals, the gradual, age-dependent collagen glycation is impaired and accelerated by two- or threefold compared with that of non-DM individuals (63). Furthermore, hyperglycemia leads to the formation of advanced glycation end products (AGEs), such as carboxymethyllysine and pentosidine agents, that are formed via the nonenzymatic, covalent modification of free proteins by reducing sugars. AGEs act through activation of a cell surface receptor of AGE (RAGE), and have been shown to have detrimental effects in both microvascular and macrovascular endothelial cells by inducing free radical oxidation and altering endothelial function (64,65).
Hyperglycemia is also likely to contribute further to endothelial dysfunction once DM develops, and poor glycemic control is a significant predictor of CVD mortality in DM (11,33,40). Although hyperglycemia is an established CVD risk factor independent of dyslipidemia, clinical trials, such as the United Kingdom Prospective Diabetes Study (UKPDS), have not been able to demonstrate definitively that an intensive glucose lowering policy reduces CAD events (11,61,62). Thus, a focus on reducing glycemia alone does not appear sufficient to reduce the excess risk in DM, highlighting the need for aggressive treatment of other risk factors. Some oral agents (eg, thiazolidinediones [TZDs]) used to treat hyper-glycemia do significantly modify cardiovascular risk factors other than hyperglycemia (66), and may have a role in reducing the CVD burden of DM.
Other risk factors
In addition to these ‘traditional’ CVD risk factors, analyses from NHANES III recently highlighted a number of ‘nontraditional’ risk factors that are present significantly more frequently in DM, including high white blood cell count, low serum albumin, low glomerular filtration rate, high plasma fibrinogen and elevated CRP (21). Furthermore, 12% of people with DM had three or more nontraditional risk factors compared with only 5% of those with normal fasting glucose.
Large-scale intervention trials highlight the impact of dyslipidemia in DM. For instance, in the UKPDS, there were significant associations between increased risk of CAD and increased concentrations of LDL-C and TGs, as well as between increased risk of CAD and decreased concentrations of HDL-C (67). The diabetes intervention study and the Paris prospective study found that TGs, in particular, were significant predictors of mortality from CAD (68,69).
Nuclear receptors associated with metabolic homeostasis
Nuclear hormone receptors may interact directly with target genes to induce a variety of physiological effects, including the regulation of cholesterol, fatty acids and glucose metabolism. They include peroxisome proliferator-activated receptor-gamma and -delta (PPAR-γ and -δ), retinoid X receptor (RXR), liver X receptor and farnesoid X receptor. The PPAR family consists of three types of receptors, termed PPAR-α, PPAR-δ and PPAR-γ. PPARs mediate a large proportion of their actions by binding to peroxisome proliferator response elements (PPREs) on DNA (43). The PPREs are direct repeats of the hexameric sequence AGGTCA, separated by one or two nucleotides. In terms of PPAR function, distinct domains have been identified that have key roles in the transduction of the PPAR-induced response. The binding of ligand to the ligand-binding domain results in a conformational change in the receptor that allows transactivation of the appropriate genes. PPARs have been shown to form heterodimers with the RXR, resulting in a synergistic effect on gene transactivation (44).
PPAR-γ is a nuclear hormone receptor that comprises an agonist-dependent activation domain (AF-2), DNA binding domain and agonist-independent activation domain (AF-1). The cloning of PPAR-γ ultimately leads to the observation that it has an important regulatory role in adipogenesis. PPAR-γ is highly expressed in adipose tissue and its overexpression in fibroblasts results in differentiation of fibroblasts into adipocytes (43,44). Upon the binding of the agonists, PPAR-γ heterodimerizes with RXR-α and activates the transcription of target genes through the binding of the PPRE.
Activation of PPAR-γ has profound effects on the myocardium and major cells of the vasculature (31,43). Firstly, PPAR-γ ligands inhibit inflammation directly in vascular cells, as well as indirectly through regulation of gene expression in adipose tissue (43). They also block vascular smooth muscle cell growth and migration in human atheroma, migration of monocytes and also promote reverse cholesterol transport. PPAR-γ ligands also inhibit endothelial cell growth and movement, thus demonstrating antiangiogenic properties (43).
At present, information is not available regarding the long-term effects of PPAR-γ activation on CVD risk and DM complications. However, a number of clinical studies have shown that its synthetic agonists, TZDs, may improve glucose tolerance by enhancing insulin sensitivity and restoring the function of β-cells in diabetic subjects (26,70–74). TZDs, also termed the ‘glitazones’, include agents such as troglitazone, pioglitazone and rosiglitazone. There is a strong correlation between the TZD-PPAR-γ interaction and antidiabetic action of TZDs; the relative potency of TZDs for binding to PPAR-γ and activation of PPAR-γ in vitro correlates well with their antidiabetic potency in vivo (75). Patients with a dominant negative mutation in the PPAR-γ gene show severe hyperglycemia, which provides a genetic link between PPAR-γ and type II DM (76). TZDs stimulate adipocyte differentiation, preferentially generating smaller adipocytes that are more sensitive to insulin, producing less FFAs, TNF-α and leptin (77,78). Although the antidiabetic action of PPAR-γ agonists is well established, there is disagreement about the mechanism proposed to explain how these agonists affect glucose metabolism. Improved glucose homeostasis may be achieved either by systemic insulin sensitization or by direct action of PPAR-γ on the transcription of genes involved in the glucose disposal. Evidence supporting the direct action of PPAR-γ on glucose metabolism has been reported. TZDs increase the expression of insulin receptor substrate (IRS)-1 (79), IRS-2 (80), the p85 subunit of phosphatidylinositol 3-kinase (81) and the Cbl-associated protein (82,83). These results are in line with the fact that TZDs increase insulin-stimulated glucose uptake in L6 myotubes (84) and in cultured human skeletal muscle cells (85,86). In addition to the insulin-sensitizing effects in peripheral tissues, PPAR-γ is known to increase the glucose-sensing ability of pancreatic β-cells (46,87–89). TZDs can reduce hepatic glucose production and increase glycogen synthesis in diabetic animal models, although controversial results have been reported.
THERAPEUTIC APPROACHES FOR PREVENTION OF CARDIOVASCULAR COMPLICATIONS IN TYPE II DM
Diabetic status appears to confer an exceptionally high risk for macrovascular disease, and there is a consensus that only a portion of patients with DM are optimally treated with a comprehensive plan that includes aggressive lipid and glycemic control, blood pressure-lowering, body mass index (BMI) reduction through diet and increased physical activity, and smoking cessation.
Therapies to lower lipids and blood pressure are proven interventions to reduce CVD (Table 3). There is strong evidence that acetylsalicylic acid (90,91), statins (92,93) and angiotensin-converting enzyme (ACE) inhibitors (94) reduce the risk of death from CVD in patients with DM. Additional therapies to the tight blood glucose control with other antihypertensive agents and fibrates have also been added, targeting the coexisting hypertension and hypertriglyceridemia, which is also widely prevalent in type II DM (95,96).
TABLE 3.
Therapies to reduce cardiovascular disease in diabetes mellitus
|
Other interventions, specifically weight loss, increased exercise and antidiabetic drugs such as metformin and acarbose, have been shown to prevent diabetes in patients with impaired glucose tolerance, but whether CVD is also prevented in this high-risk group is not yet known (97,98).
With respect to nonmedical therapies, coronary artery bypass grafting is associated with a greater success than is percutaneous transluminal coronary angioplasty (PTCA) for patients with multivessel CAD (99). For persons with diabetes undergoing PTCA, the combination of a stent and glycoprotein IIb/IIIa inhibitor significantly reduces restenosis rates and serious morbidity, compared with PTCA alone. Trials using drug-eluting stents in diabetes are currently underway. Other interventions, such as smoking cessation and blood glucose control, were deemed ‘likely to be beneficial’ for lowering the risk of heart disease, according to the evidence-based guidelines (100).
Use of acetylsalicylic acid
Low-dose daily acetylsalicylic acid is being advocated to prevent a first MI. The United States Preventive Service Task Force strongly recommends acetylsalicylic acid chemoprevention for adults who are at increased risk for CAD, including people with diabetes, based on a meta-analysis of five primary prevention trials that included the seminal United States Physicians’ Health Study (101). The American Diabetes Association also recommends acetylsalicylic acid for the primary prevention of heart disease in diabetic patients older than 30 years with no contraindications (102). The American Heart Association Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2000 Update (103) call for low-dose acetylsalicylic acid for primary prevention in patients with a 10-year risk of heart disease that exceeds 10%.
Lifestyle modification to prevent diabetes
Therapeutic lifestyle changes, including reducing intake of saturated fats and cholesterol, increasing intake of fibre, reducing body weight and increasing physical activity, represent the first step for CAD risk reduction in the recommendations of both the NCEP and the Joint European Societies (104,105). Lifestyle modifications also play an important role in strategies to prevent and treat diabetes (106–108). The benefits of lifestyle modification in risk reduction can be significant; for example, it is estimated that a 10% reduction in BMI in patients with type II DM can reduce systolic blood pressure by 15 mmHg to 20 mmHg, total cholesterol by 1 mmol/L to 2 mmol/L and HbA1c by 1% to 2%. Two large studies (109,110) have recently shown that such lifestyle intervention is highly successful in preventing development of type II DM.
Use of ACE inhibitors
The relationship between the renin-angiotensin-aldosterone system and diabetic macrovascular and microvascular disease is well established (111).Vascular angiotensin plays an important role in the long-term regulation of the blood vessel function and structure. Angiotensin stimulates vascular smooth muscle cell growth via the induction of proto-oncogene and autocrine growth factor gene expressions (112). Angiotensin II acts principally through the angiotensin type 1 receptors leading to a range of harmful effects that culminates in vascular inflammation, atherosclerosis (113) and oxidative stress (114–118).
ACE inhibitors have been showed to have long-term benefits in CVD. These drugs have been considered for the treatment of hypertension in diabetic patients with heart failure, previous MI or proteinuria. Moreover, pharmacological interventions with ACE inhibitors lead to the accumulation of bradykinin and suppression of angiotensin II (119).
The Heart Outcomes Prevention Evaluation (HOPE) study (120) demonstrated the significant benefits of ACE inhibition in patients with documented coronary disease. This study assessed the effects of treatment with the ACE inhibitor ramipril, versus placebo, in 9297 patients, who had evidence of vascular disease or diabetes plus one additional cardiovascular risk factor, and who did not have left ventricular dysfunction or heart failure. Treatment with ramipril resulted in reduced rates of death from cardiovascular causes, MI, stroke, death from any cause, revascularization procedures, cardiac arrest, heart failure and complications related to diabetes. In terms of heart failure, ramipril treatment reduced the risk of new-onset heart failure by 23% (120). A substudy of the HOPE trial, the Microalbuminuria, Cardiovascular, and Renal Outcomes (MICRO)-HOPE study (121), examined whether ramipril can lower the risks of CVD and renal disease in patients with diabetes. The analysis included 3577 patients with diabetes who had been included in the HOPE study. The independent safety and monitoring board stopped the MICRO-HOPE study early (after 4.5 years) because ramipril therapy demonstrated a consistent benefit compared with placebo in this patient population. Specifically, it reduced the risk of total mortality by 24%, MI by 22%, stroke by 33%, cardiovascular death by 37% and revascularization by 17% (122). The HOPE and MICRO-HOPE studies provide convincing evidence that ACE inhibition can lower the risk of new-onset heart failure in patients with diabetes. In patients with symptomatic heart failure, there is a wealth of data demonstrating the benefits of ACE inhibitor therapy. Garg and Yusuf (122) analyzed 32 randomized, controlled trials of ACE inhibitor therapy in patients with symptomatic congestive heart failure and found that ACE inhibitor treatment resulted in a 23% reduction in mortality.
A more recent analysis of major clinical trials of ACE inhibitors further shows that patients with heart failure who have diabetes experience a benefit from therapy with ACE inhibitors similar to that of their nondiabetic counterparts (123).
Use of lipid-regulating agents (statins and fibrates)
Randomized clinical trials have demonstrated that lipid-regulating agents (statins or fibrates) significantly reduce the risk of cardiovascular events in subjects with diabetes and dyslipidemia.
The inhibitors of HMG-CoA reductase, known generically as statins, are the recommended first-line therapy for patients with dyslipidemia (11,124). These drugs inhibit the rate-limiting step in cholesterol biosynthesis, the conversion of HMG-CoA to mevalonate. This reduces the availability of cholesterol for packaging into lipoproteins, such as VLDL and LDL. Consequently, upregulation of hepatic LDL receptors also occurs, leading to a further fall in plasma LDL-C. It is believed that this well-documented fall in LDL-C underpins most of the beneficial effect of the statins. Clinical trials of statins have shown convincingly that they decrease overall morbidity and mortality from CAD by one-third, with a similar decrease in risk of nonhemorrhagic stroke and a decrease in all-cause mortality of almost one-quarter (125). For instance, the Scandinavian Simvastatin Survival Study (4S) showed that simvastatin therapy reduced CAD events by 55% in people with DM (126).
Pooled data from the Cholesterol and Recurrent Events (CARE) and Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) studies of pravastatin therapy also revealed significantly reduced CAD events in people with DM (127). The Collaborative Atorvastatin Diabetes Study (CARDS) was halted two years early, because patients on statin therapy had significant reductions in acute coronary events by 36% and stroke by 48% (128). While the American College of Physicians has recommended the widespread use of statin therapy to prevent CVD in patients with type II DM (129,130), not all clinicians agree that statins should be used in all patients with the disorder (131). Although statins are effective at lowering LDL-C (by approximately 20% to 25%), their effects on TGs and HDL-C are less impressive, with approximately 10% to 15% decreases in TGs and only approximately 5% increases in HDL-C, and a substantial ‘residual’ cardiovascular risk may remain without appropriate attention to these risk factors (125,132–134).
Use of PPAR-γ activators
PPAR-γ activation – effects on lipid profile
Insulin resistance results in increased levels of plasma TGs, decreased HDL-C and a predominance of highly atherogenic, small, dense, LDL-C particles, as mentioned previously (22–25). The TZDs have beneficial effects in attenuating this complex dyslipidemic state. Typical quantitative changes have been described in studies using pioglitazone (135–139) and rosiglitazone (140). In dyslipidemic patients, TG levels did not change significantly from baseline with rosiglitazone therapy, whereas total cholesterol, HDL-C and LDL-C levels increased at doses greater than 4 mg. However, LDL-C levels stabilized after approximately three months of treatment with 8 mg of rosiglitazone, whereas HDL-C levels continued to increase during 1.5 years of follow-up, leading to an overall decrease in the LDL-C to HDL-C ratio. The clinical significance of the small increase in LDL-C is unclear. As with troglitazone, the increase in LDL-C concentration with rosiglitazone and pioglitazone occurs mainly in the larger, more buoyant, apparently less atherogenic subfraction of LDL-C (140,141). Rosiglitazone also reduces plasma levels of the atherogenic, small, dense LDL-C and increases levels of the atheroprotective large HDL-C (70). Both rosiglitazone and pioglitazone decrease circulating FFA levels, an effect that may relate to improved insulin sensitivity (140).
These effects on lipid abnormalities may not be a class effect of TZDs, because pioglitazone and rosiglitazone have distinct effects on lipoprotein metabolism, which may have important clinical implications for cardiovascular risk reduction in patients with type II DM. In a retrospective study (140) from primary care practices, pioglitazone demonstrated significantly greater benefits on blood lipid parameters (TGs, total cholesterol and LDL-C) relative to rosiglitazone, while glycemic control (as measured by HbA1c) was comparable. Furthermore, recent results from a randomized, prospective, double-blind study comparing the two agents showed that, relative to rosiglitazone, pioglitazone significantly improved TGs, HDL-C and LDL particle concentration and size. Non-HDL-C remained stable with pioglitazone, but increased with rosiglitazone.
PPAR-γ activation – effects on endothelial dysfunction and inflammation
The TZDs have salutary effects on endothelial dysfunction, a key factor in the pathogenesis of atherogenesis (141). For example, expression of endothelin-1 (ET-1), a potent, endothelial-derived vasoconstrictor compound thought to be involved in the pathogenesis of numerous complications of diabetes, is regulated by TZD action. In the kidney, glomerular endothelial cells, mesangial cells and epithelial cells secrete ET-1, and alterations in urinary and renal ET-1 concentrations are associated with the development of diabetic nephropathy (142).
Patients with type II DM treated with TZDs have shown improvements in other nonlipid cardiovascular risk markers, either directly by affecting vascular smooth walls and cells involved in the atherogenic process or indirectly by improving insulin sensitivity. Markers of inflammation, coagulation and thrombosis, blood pressure and urinary albumin to creatinine ratio (a measure of microalbuminuria) have all been improved with TZD therapy. Both rosiglitazone and pioglitazone significantly improved the albumin to creatinine ratio, accompanied by reductions in blood pressure, in patients with type II DM compared with comparator oral agents (143).
In a study of 45 normotensive, euglycemic type II DM patients with microalbuminuria compared with 30 healthy controls, pioglitazone reduced urinary albumin excretion and urinary ET-1 excretion, neither of which was affected by glibenclamide or voglibose (143).
In patients with type II DM, TZDs (rosiglitazone or pioglitazone) have been shown to reduce CRP levels independent of any effect on glycemia (144,145). A recent study by Pampanelli et al (146) showed that six to 12 months of pioglitazone therapy also significantly improved coagulation and thrombosis parameters in patients with type II DM independent of glucose control – platelets, vWF (a marker of endothelial dysfunction) and PAI-1 were reduced, whereas antithrombin III and fibrinogen were increased. No significant changes were seen in a gliclazide comparator group and between-group differences were significant for vWF, PAI-1 and anti-thrombin III. Satoh et al (145) also demonstrated that three months of pioglitazone therapy significantly reduced pulse wave velocity (a marker of vascular damage and also a predictor of mortality in DM). Furthermore, in a study by Koshiyama et al (147), pioglitazone decreased the carotid arterial intima-media wall thickness (an early sign of atherosclerotic change) in patients with type II DM.
PPAR-γ activation – effect on blood pressure
Studies conducted on laboratory animals suggest that the TZDs may be useful in the prevention and treatment of hypertension, particularly when associated with insulin resistance (148). This finding is not surprising when understood in the context of the role of insulin resistance in the genesis of hypertension. The blood pressure-lowering effects of the TZDs are relevant because patients with type II DM are twice as likely to be hypertensive as individuals who do not have DM (124). In an open-label, 52-week comparison study (149), rosiglitazone, but not glibenclamide, produced statistically significant reductions in systolic, diastolic and mean arterial blood pressures. In another study (125), decreases in mean arterial pressure with the use of troglitazone correlated significantly with reductions in plasma insulin, suggesting a link between improvement of insulin sensitivity and blood pressure reduction. Other potential mechanisms of TZD-induced blood pressure reduction may include improved endothelium-dependent vasodilation, decrease in calcium influx and calcium sensitivity of the contractile apparatus, and inhibition of ET-1 expression and secretion (126).
PPAR-γ activation – effect on pancreatic β-cell function
There is growing evidence of the usefulness of the TZDs in preserving pancreatic β-cell function. In an observational, nested, case-control study, 28 patients with type II DM who had not responded to metformin-sulfonylurea combination therapy had a meal-stimulated C-peptide level documented before the addition of a TZD to their regimen. The control group consisted of 26 age-, BMI- and glycemia-matched patients with type II DM who also had a meal-stimulated C-peptide level documented before adding metformin to a failing sulfonylurea monotherapy regimen. The mean C-peptide level in the TZD group showed a small but significant increase, whereas C-peptide levels remained unchanged in controls. The C-peptide to glucose ratio also increased significantly in the TZD group, whereas it remained unchanged in controls (150). These findings suggest that TZD therapy induced recovery of pancreatic β-cell function independently of the correction of glucose toxicity.
A recent report assessed the effects of rosiglitazone on pancreatic β-cell function. Twenty patients with type II DM were randomly assigned to rosiglitazone, 4 mg twice daily, or placebo for 13 weeks. Fasting plasma glucose was reduced and insulin sensitivity was increased by rosiglitazone. First-phase insulin secretion and insulin secretory capacity were unaffected; however, glucose-entrained insulin secretion was significantly increased (151). These data suggest that short-term treatment with rosiglitazone may increase the ability of the β-cell to sense and respond to physiological changes in glucose levels.
Combination therapy of statins and PPAR-γ activators
A novel therapeutic approach to reduce CVD risk in type II DM may be to use lower-dose statins in combination with TZDs. This has the potential to target multiple cardiovascular risk factors, while also treating the primary symptom of hyper-glycemia. As described above, TZDs produce changes in several cardiovascular risk factors associated with the insulin resistance syndrome, including correcting diabetic dyslipidemia, improving fibrinolysis and decreasing carotid artery intima-medial thickness (26) and, as such, TZD therapy provides a novel approach in the prevention of cardiovascular complications in patients with type II DM. Whereas statins produce a marked reduction in LDL-C and a moderate reduction in TGs, with a slight increase in HDL-C (125), pioglitazone has a particularly beneficial effect on TGs and HDL-C. Although TZDs increase LDL-C, they induce a favourable change in the LDL particle size and susceptibility to oxidation as shown for trogli-tazone by Tack et al (152). This has been confirmed more recently for pioglitazone in nondiabetic patients with hypertension (in whom the prevalence of atherogenic dense LDL is similar to that in patients with type II DM), as well as in patients with type II DM (153,154). These qualitative changes may have a beneficial effect on cardiovascular risk profile and compensate for a small increase in LDL-C.
Therefore, statins and pioglitazone would appear to have complementary effects on lipid profiles, suggesting a rationale for their use in combination therapy. A recent study by Lewin et al (155) provides strong support for this rationale – the addition of low-dose simvastatin to stable pioglitazone or rosiglitazone therapy (pooled data) resulted in improvements in all lipid parameters measured, including LDL-C. TZDs also have beneficial effects on other markers of cardiovascular risk, which suggest that combination statin/TZD therapy could address the multiple aspects of diabetic dyslipidemia, as well as having the potential to reduce the incidence of macrovascular events further.
Recently presented data indicate that pioglitazone and simvastatin may act synergistically to lower levels of inflammatory cytokines, including IL-6 and CRP, as well as optimizing patients’ lipid profiles (156–158). The innovative Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) is currently assessing the effect of pioglitazone on the secondary prevention of macrovascular events in patients with type II DM, who have a history of macrovascular disease and are at high risk of further macrovascular events (159–161). In this study, pioglitazone is being used as ‘add-on’ therapy to current treatment, which is continuously optimized throughout the trial to allow patients to receive the best possible therapy.
One-third of patients enrolled were at high vascular risk based on HDL-C (32.6%) or TG (36.0%) levels, whereas two-thirds were at risk based on raised systolic and/or diastolic blood pressure, and, at baseline, one-half of the patients were taking lipid-lowering medication (with 42.9% of all patients on statins). It is anticipated that this study will provide important insights into the potential benefits of statin and TZD cotherapy (159–161).
CONCLUSIONS
Current research data indicate that insulin resistance, the metabolic syndrome and type II DM are inextricably linked with CVD, as apparent from the increased levels of CVD morbidity and mortality and from the presence of a complex array of CVD risk markers. Targeting multiple markers of CVD risk hopefully offers the best chance of improving CVD outcomes.
It is clear that TZDs, and pioglitazone in particular, can provide cardiovascular benefits in improving glucose levels, and probably lipid profiles. Ongoing studies should generate important insights into whether pioglitazone can play a larger role in the management of CVD and type II DM, especially in high-risk cardiovascular patients with the disorder.
REFERENCES
- 1.Underwood JCE. General and Systematic Pathology. Edinburgh: Churchill Livingstone; 1992. [Google Scholar]
- 2.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 3.Williams G, Pickup JC. Handbook of Diabetes. Oxford: Blackwell Science Inc; 1998. [Google Scholar]
- 4.Geiss LS, Herman WH, Smith PJ. Diabetes. 2. National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995. Mortality in non-insulin-dependent diabetes; pp. 233–257. NIH Publication No. 95–1468. [Google Scholar]
- 5.Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–81. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 6.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: An epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 7.de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: The Hoorn Study. Diabetologia. 1999;42:926–391. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: The Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Wingard DL, Barrett-Connor E. Diabetes in America. 2. National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995. Heart Disease and Diabetes; pp. 429–48. NIH Publication No. 95–1468. [Google Scholar]
- 10.Mukamal KJ, Nesto RW, Cohen MC, et al. Impact of diabetes on long-term survival after acute myocardial infarction: Comparability of risk with prior myocardial infarction. Diabetes Care. 2001;24:1422–7. doi: 10.2337/diacare.24.8.1422. [DOI] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 12.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Part 1, Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. [Google Scholar]
- 14.Laakso M. Insulin resistance and cardiovascular disease. Br J Diabetes Vasc Dis. 2002;2(Suppl 1):S9–11. [Google Scholar]
- 15.Kendall DM, Sobel BE, Coulston AM, et al. The insulin resistance syndrome and coronary artery disease. Coron Artery Dis. 2003;14:335–48. doi: 10.1097/01.mca.0000076512.29238.2a. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Sharrett AR, Klein BE, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: The atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–34. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 17.Mykkanen L, Kuusisto J, Pyorala K, Laakso M. Cardiovascular disease risk factors as predictors of type 2 (non-insulin-dependent) diabetes mellitus in elderly subjects. Diabetologia. 1993;36:553–9. doi: 10.1007/BF02743273. [DOI] [PubMed] [Google Scholar]
- 18.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 19.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: Analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004;14:686–95. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Berthezène F. Diabetic dyslipidaemia. Br J Diabetes Vasc Dis. 2002;2(Suppl 1):S12–17. [Google Scholar]
- 23.Reaven GM. Insulin resistance: Why is it important to treat? Diabetes Metab. 2001;27:247–53. [PubMed] [Google Scholar]
- 24.Bays H. Atherogenic dyslipidaemia in type 2 diabetes and metabolic syndrome: Current and future treatment options. Br J Diabetes Vasc Dis. 2003;3:356–60. [Google Scholar]
- 25.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med. 2003;115(Suppl 8A):24S–28S. doi: 10.1016/j.amjmed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca VA. Management of diabetes mellitus and insulin resistance in patients with cardiovascular disease. Am J Cardiol. 2003;92:50J–60J. doi: 10.1016/s0002-9149(03)00616-7. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW, Anderson KM, Kannel WB. Epidemiology of diabetes mellitus in the elderly. The Framingham Study. Am J Med. 1986;80:3–9. doi: 10.1016/0002-9343(86)90532-2. [DOI] [PubMed] [Google Scholar]
- 28.Aoun S, Blacher J, Safar ME, Mourad JJ. Diabetes mellitus and renal failure: Effects on large artery stiffness. J Hum Hypertens. 2001;15:693–700. doi: 10.1038/sj.jhh.1001253. [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft JR, Webb DJ, Wilkinson IB. Arterial stiffness, hypertension and diabetes mellitus. J Hum Hypertens. 2000;14:377–80. doi: 10.1038/sj.jhh.1001023. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa O, Onuma T, Kubo S, Mitsuhashi N, Muramatsu C, Kawamori R. Brachial-ankle pulse wave velocity and symptomatic cerebral infarction in patients with type 2 diabetes: A cross-sectional study. Cardiovasc Diabetol. 2003;2:10. doi: 10.1186/1475-2840-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: A comparison of diabetic and nondiabetic subjects. Diabetes Care. 2003;26:2133–8. doi: 10.2337/diacare.26.7.2133. [DOI] [PubMed] [Google Scholar]
- 32.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92:10J–17J. doi: 10.1016/s0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh WA, Lyon CJ, Quinones MJ. Insulin resistance and the endothelium. Am J Med. 2004;117:109–17. doi: 10.1016/j.amjmed.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Makimattila S, Yki-Jarvinen H. Endothelial dysfunction in human diabetes. Curr Diab Rep. 2002;2:26–36. doi: 10.1007/s11892-002-0054-x. [DOI] [PubMed] [Google Scholar]
- 35.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim HA, El-Meligi AA, Abdel-Hamid M, Elhendy A. Relations between von Willebrand factor, markers of oxidative stress and microalbuminuria in patients with type 2 diabetes mellitus. Med Sci Monit. 2004;10:CR85-9. [PubMed] [Google Scholar]
- 37.Zareba W, Pancio G, Moss AJ, et al. Increased level of von Willebrand factor is significantly and independently associated with diabetes in postinfarction patients. Thromb Haemost. 2001;86:791–9. [PubMed] [Google Scholar]
- 38.Henry RM, Ferreira I, Kostense PJ, et al. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis. 2004;174:49–56. doi: 10.1016/j.atherosclerosis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Ravikumar R, Deepa R, Shanthirani C, Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]) Am J Cardiol. 2002;90:702–7. doi: 10.1016/s0002-9149(02)02593-6. [DOI] [PubMed] [Google Scholar]
- 40.Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303–9. doi: 10.2337/diab.44.11.1303. [DOI] [PubMed] [Google Scholar]
- 41.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: Altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–47. [PubMed] [Google Scholar]
- 42.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 43.Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: Implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- 44.Einhorn D, Aroda VR, Henry RR. Glitazones and the management of insulin resistance: What they do and how might they be used. Endocrinol Metab Clin North Am. 2004;33:595–616. doi: 10.1016/j.ecl.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Haffner SM. Pre-diabetes, insulin resistance, inflammation and CVD risk. Diabetes Res Clin Pract. 2003;61(Suppl 1):S9–S18. doi: 10.1016/s0168-8227(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 46.Nesto R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet Med. 2004;21:810–7. doi: 10.1111/j.1464-5491.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;144:537–47. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 49.Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109:1729–34. doi: 10.1161/01.CIR.0000124718.99562.91. [DOI] [PubMed] [Google Scholar]
- 50.Ceriello A, Falleti E, Bortolotti N, et al. Increased circulating intercellular adhesion molecule-1 levels in type II diabetic patients: The possible role of metabolic control and oxidative stress. Metabolism. 1996;45:498–501. doi: 10.1016/s0026-0495(96)90226-7. [DOI] [PubMed] [Google Scholar]
- 51.Cominacini L, Fratta Pasini A, Garbin U, et al. Elevated levels of soluble E-selectin in patients with IDDM and NIDDM: Relation to metabolic control. Diabetologia. 1995;38:1122–4. doi: 10.1007/BF00402185. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 53.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 54.Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89:1092–103. doi: 10.1161/hh2401.101272. [DOI] [PubMed] [Google Scholar]
- 55.Lim HS, Blann AD, Lip GY. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: Relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–8. doi: 10.1161/01.CIR.0000129773.70647.94. [DOI] [PubMed] [Google Scholar]
- 56.Tayebjee MH, Macfadyen RJ, Lip GY. Extracellular matrix biology: A new frontier in linking the pathology and therapy of hypertension? J Hypertens. 2003;21:2211–8. doi: 10.1097/01.hjh.0000098178.36890.81. [DOI] [PubMed] [Google Scholar]
- 57.Tayebjee MH, Lim HS, Nadar S, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinase-1 is a marker of diastolic dysfunction using tissue doppler in patients with type 2 diabetes and hypertension. Eur J Clin Invest. 2005;35:8–12. doi: 10.1111/j.1365-2362.2005.01438.x. [DOI] [PubMed] [Google Scholar]
- 58.Maxwell PR, Timms PM, Chandran S, Gordon D. Peripheral blood level alterations of TIMP-1, MMP-2, and MMP-9 in patients with type 1 diabetes. Diabet Med. 2001;18:777–80. doi: 10.1046/j.1464-5491.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- 59.Van Bortel LM, Struijker-Boudier HA, Safar ME. Pulse pressure, arterial stiffness, and drug treatment of hypertension. Hypertension. 2001;38:914–21. doi: 10.1161/hy1001.095773. [DOI] [PubMed] [Google Scholar]
- 60.Tayebjee MH, Lim HS, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 in type 2 diabetes: Effect of 1 year’s cardiovascular risk reduction therapy. Diabetes Care. 2004;27:2049–51. doi: 10.2337/diacare.27.8.2049. [DOI] [PubMed] [Google Scholar]
- 61.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 62.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 63.Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1991;87:1910–5. doi: 10.1172/JCI115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dyer DG, Dunn JA, Thorpe SR, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCance DR, Dyer DG, Dunn JA, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–8. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin JJ, Rothman J, Farag A, McFarlane SI, Sowers JR. Role of oral anti-diabetic agents in modifying cardiovascular risk factors. Minerva Med. 2003;94:401–8. [PubMed] [Google Scholar]
- 67.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fontbonne A, Eschwege E, Cambien F, et al. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989;32:300–4. doi: 10.1007/BF00265546. [DOI] [PubMed] [Google Scholar]
- 69.Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: The Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577–83. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 70.Florkowski CM. Management of co-existing diabetes mellitus and dyslipidemia: Defining the role of thiazolidinediones. Am J Cardiovasc Drugs. 2002;2:15–21. doi: 10.2165/00129784-200202010-00003. [DOI] [PubMed] [Google Scholar]
- 71.Gilling L, Suwattee P, DeSouza C, Asnani S, Fonseca V. Effects of the thiazolidinediones on cardiovascular risk factors. Am J Cardiovasc Drugs. 2002;2:149–56. doi: 10.2165/00129784-200202030-00002. [DOI] [PubMed] [Google Scholar]
- 72.Greenberg AS. The expanding scope of the metabolic syndrome and implications for the management of cardiovascular risk in type 2 diabetes with particular focus on the emerging role of the thiazolidinediones. J Diabetes Complications. 2003;17:218–28. doi: 10.1016/s1056-8727(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 73.Stolar MW, Chilton RJ. Type 2 diabetes, cardiovascular risk, and the link to insulin resistance. Clin Ther. 2003;25(Suppl B):B4–31. doi: 10.1016/s0149-2918(03)80240-0. [DOI] [PubMed] [Google Scholar]
- 74.Zimmet P. Addressing the insulin resistance syndrome: A role for the thiazolidinediones. Trends Cardiovasc Med. 2002;12:354–62. doi: 10.1016/s1050-1738(02)00187-1. [DOI] [PubMed] [Google Scholar]
- 75.Berger J, Bailey P, Biswas C, et al. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: Binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996;137:4189–95. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 76.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 77.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 78.Spiegelman BM. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 79.Iwata M, Haruta T, Usui I, et al. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator –activated receptor-gamma. Diabetes. 2001;50:1083–92. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- 80.Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. FASEB J. 2001;15:215–220. doi: 10.1096/fj.00-0020com. [DOI] [PubMed] [Google Scholar]
- 81.Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor gamma with rosiglitazone (BRL 49653) in human adipocytes. Biochem Biophys Res Commun. 1999;265:265–71. doi: 10.1006/bbrc.1999.1657. [DOI] [PubMed] [Google Scholar]
- 82.Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: Peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci U S A. 1998;95:14751–6. doi: 10.1073/pnas.95.25.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baumann CA, Chokshi N, Saltiel AR, Ribon V. Cloning and characterization of a functional peroxisome proliferator activator receptor-gamma-responsive element in the promoter of the CAP gene. J Biol Chem. 2000;275:9131–5. doi: 10.1074/jbc.275.13.9131. [DOI] [PubMed] [Google Scholar]
- 84.Zhang B, Szalkowski D, Diaz E, Hayes N, Smith R, Berger J. Potentiation of insulin stimulation of phosphatidylinositol 3-kinase by thiazolidinedione-derived antidiabetic agents in Chinese hamster ovary cells expressing human insulin receptors and L6 myotubes. J Biol Chem. 1994;269:25735–41. [PubMed] [Google Scholar]
- 85.Kausch C, Krutzfeldt J, Witke A, et al. Effects of troglitazone on cellular differentiation, insulin signaling, and glucose metabolism in cultured human skeletal muscle cells. Biochem Biophys Res Commun. 2001;280:664–74. doi: 10.1006/bbrc.2000.4216. [DOI] [PubMed] [Google Scholar]
- 86.Cha BS, Ciaraldi TP, Carter L, et al. Peroxisome proliferator-activated receptor (PPAR) gamma and retinoid X receptor (RXR) agonists have complementary effects on glucose and lipid metabolism in human skeletal muscle. Diabetologia. 2001;44:444–52. doi: 10.1007/s001250051642. [DOI] [PubMed] [Google Scholar]
- 87.Meriden T. Progress with thiazolidinediones in the management of type 2 diabetes mellitus. Clin Ther. 2004;26:177–90. doi: 10.1016/s0149-2918(04)90017-3. [DOI] [PubMed] [Google Scholar]
- 88.Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Ann Intern Med. 2001;134:61–71. doi: 10.7326/0003-4819-134-1-200101020-00014. [DOI] [PubMed] [Google Scholar]
- 89.Smith U. Thiazolidinedione-induced effects beyond glycaemic control. Br J Diabetes Vasc Dis. 2002;2(Suppl 1):S24–S27. [Google Scholar]
- 90.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy – I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 91.American Diabetes Association. Aspirin therapy in diabetes. Diabetes Care. 2003;26(Suppl 1):S87–8. doi: 10.2337/diacare.26.2007.s87. [DOI] [PubMed] [Google Scholar]
- 92.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 93.American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S83–6. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 94.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 95.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 96.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes. The Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–10. [PubMed] [Google Scholar]
- 97.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–8. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 98.Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin Sci (Lond) 1996;90:189–95. doi: 10.1042/cs0900189. [DOI] [PubMed] [Google Scholar]
- 99.Zuraw B. Bradykinin in protection against left-ventricular hypertrophy. Lancet. 2001;358:1116–8. doi: 10.1016/S0140-6736(01)06300-0. [DOI] [PubMed] [Google Scholar]
- 100.Dell’Italia LJ, Oparil S. Bradykinin in the heart: Friend or foe? Circulation. 1999;100:2305–7. doi: 10.1161/01.cir.100.23.2305. [DOI] [PubMed] [Google Scholar]
- 101.Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–48. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- 102.Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med. 2000;45:419–29. [PubMed] [Google Scholar]
- 103.Goldstein LB, Adams R, Alberts MJ, et al. American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 104.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 105.Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and other Societies on coronary prevention. Eur Heart J. 1998;19:1434–503. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]
- 106.American Diabetes Association. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25(Suppl 1):S50–60. doi: 10.2337/diacare.25.1.202. [DOI] [PubMed] [Google Scholar]
- 107.American Diabetes Association. Diabetes mellitus and exercise. Diabetes Care. 2002;25(Suppl 1):S64–8. [Google Scholar]
- 108.Jung RT. Obesity as a disease. Br Med Bull. 1997;53:307–21. doi: 10.1093/oxfordjournals.bmb.a011615. [DOI] [PubMed] [Google Scholar]
- 109.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 110.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sowers JR, Stump CS. Insights into the biology of diabetic vascular disease: What’s new? Am J Hypertens. 2004;17:2S–6S. doi: 10.1016/j.amjhyper.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 112.Dzau VJ. Vascular renin-angiotensin system and vascular protection. J Cardiovasc Pharmacol. 1993;22(Suppl 5):S1–9. doi: 10.1097/00005344-199322005-00002. [DOI] [PubMed] [Google Scholar]
- 113.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 114.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–20. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 115.Givertz MM. Manipulation of the renin-angiotensin system. Circulation. 2001;104:E14–8. doi: 10.1161/hc3001.094733. [DOI] [PubMed] [Google Scholar]
- 116.Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–45. doi: 10.1016/s0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- 117.Francis GS. ACE inhibition in cardiovascular disease. N Engl JMed. 2000;342:201–2. doi: 10.1056/NEJM200001203420309. [DOI] [PubMed] [Google Scholar]
- 118.Weber KT. Aldosterone in congestive heart failure. N Engl JMed. 2001;345:1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 119.Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med. 2000;45:419–29. [PubMed] [Google Scholar]
- 120.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 121.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 122.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–6. [PubMed] [Google Scholar]
- 123.Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: A meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–38. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 124.Diep QN, El Mabrouk M, Cohn JS, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: Role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 125.Rosenstock J, Raskin P. Hypertension in diabetes mellitus. Cardiol Clin. 1988;6:547–60. [PubMed] [Google Scholar]
- 126.Simonson DC. Etiology and prevalence of hypertension in diabetic patients. Diabetes Care. 1988;11:821–7. doi: 10.2337/diacare.11.10.821. [DOI] [PubMed] [Google Scholar]
- 127.American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):S68–71. doi: 10.2337/diacare.27.2007.s68. [DOI] [PubMed] [Google Scholar]
- 128.Durrington P. Clinical trials of lipid-lowering medication in diabetes. Br J Diabetes Vasc Dis. 2003;3:217–20. [Google Scholar]
- 129.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 130.Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: The Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–900. doi: 10.1161/01.cir.102.16.1893. [DOI] [PubMed] [Google Scholar]
- 131.Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 132.Snow V, Aronson MD, Hornbake ER, Mottur-Pilson C, Weiss KB Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Lipid control in the management of type 2 diabetes mellitus: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2004;140:644–9. doi: 10.7326/0003-4819-140-8-200404200-00012. [DOI] [PubMed] [Google Scholar]
- 133.Vijan S, Hayward RA American College of Physicians. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: Background paper for the American College of Physicians. Ann Intern Med. 2004;140:650–8. doi: 10.7326/0003-4819-140-8-200404200-00013. [DOI] [PubMed] [Google Scholar]
- 134.Garg A. Statins for all patients with type 2 diabetes: Not so soon. Lancet. 2004;364:641–2. doi: 10.1016/S0140-6736(04)16907-9. [DOI] [PubMed] [Google Scholar]
- 135.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: A 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 136.Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P. A long-term comparison of pioglitazone and gliclazide in patients with Type 2 diabetes mellitus: A randomized, double-blind, parallel-group comparison trial. Diabet Med. 2005;22:399–405. doi: 10.1111/j.1464-5491.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 137.Derosa G, Cicero AF, Gaddi A, et al. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with glimepiride: A twelve-month, multicenter, double-blind, randomized, controlled, parallel-group trial. Clin Ther. 2004;26:744–54. doi: 10.1016/s0149-2918(04)90074-4. [DOI] [PubMed] [Google Scholar]
- 138.Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with Type 2 diabetes. Diabetes Care. 2004;27:141–7. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 139.Herz M, Johns D, Reviriego J, et al. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naive patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1074–95. doi: 10.1016/s0149-2918(03)80068-1. [DOI] [PubMed] [Google Scholar]
- 140.Boyle PJ, King AB, Olansky L, et al. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: A retrospective review of randomly selected medical records. Clin Ther. 2002;24:378–96. doi: 10.1016/s0149-2918(02)85040-8. [DOI] [PubMed] [Google Scholar]
- 141.Perez A, Khan M, Johnson T, Karunaratne M. Effects of pioglitazone on lipid subspecies and subparticle profiles: Results from a double-blind, randomized study of pioglitazone HCl vs placebo in reducing or eliminating insulin requirement in subjects with type 2 diabetes. Diabetes. 2004;52:A141. (Abst) [Google Scholar]
- 142.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 143.Hopfner RL, Gopalakrishnan V. Endothelin: Emerging role in diabetic vascular complications. Diabetologia. 1999;42:1383–94. doi: 10.1007/s001250051308. [DOI] [PubMed] [Google Scholar]
- 144.Rajagopalan R, Khan M. Effect of pioglitazone on metabolic syndrome risk markers in patients with type 2 diabetes: Analysis from four randomized trials. Diabetes. 2004;52:A142–3. (Abst) [Google Scholar]
- 145.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–84. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 146.Satoh N, Ogawa Y, Usui T, et al. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care. 2003;26:2493–9. doi: 10.2337/diacare.26.9.2493. [DOI] [PubMed] [Google Scholar]
- 147.Pampanelli S, Rinaldi T, Perriello G, Brunetti P. Effects of pioglitazone on coagulation and thrombosis in comparison to gliclazide in patients with type 2 diabetes. Diabetes. 2004;52:A140. (Abst) [Google Scholar]
- 148.Fullert S, Schneider F, Haak E, et al. Effects of pioglitazone in nondiabetic patients with arterial hypertension: A double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2002;87:5503–6. doi: 10.1210/jc.2002-020963. [DOI] [PubMed] [Google Scholar]
- 149.Yosefy C, Magen E, Kiselevich A, Priluk R, London D, Volchek L, Viskoper RJ., Jr Rosiglitazone improves, while Glibenclamide worsens blood pressure control in treated hypertensive diabetic and dyslipidemic subjects via modulation of insulin resistance and sympathetic activity. J Cardiovasc Pharmacol. 2004;44:215–22. doi: 10.1097/00005344-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 150.Ovalle F, Bell DS. Clinical evidence of thiazolidinedione-induced improvement of pancreatic beta-cell function in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2002;4:56–59. doi: 10.1046/j.1463-1326.2002.00183.x. [DOI] [PubMed] [Google Scholar]
- 151.Juhl CB, Hollingdal M, Porksen N, Prange A, Lonnqvist F, Schmitz O. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: Evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88:3794–800. doi: 10.1210/jc.2002-021181. [DOI] [PubMed] [Google Scholar]
- 152.Laakso M, Lehto S, Penttila I, Pyorala K. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non-insulin-dependent diabetes. Circulation. 1993;88:1421–30. doi: 10.1161/01.cir.88.4.1421. [DOI] [PubMed] [Google Scholar]
- 153.Winkler K, Friedrich I, Baumstark MW, Wieland H, Marz W. Pioglitazone reduces atherogenic dense low density lipoprotein (LDL) particles in patients with type 2 diabetes mellitus. Br J Diabetes Vasc Dis. 2002;2:143–8. [Google Scholar]
- 154.Winkler K, Konrad T, Fullert S, et al. Pioglitazone reduces atherogenic dense LDL particles in nondiabetic patients with arterial hypertension: A double-blind, placebo-controlled study. Diabetes Care. 2003;26:2588–94. doi: 10.2337/diacare.26.9.2588. [DOI] [PubMed] [Google Scholar]
- 155.Lewin AJ, Kipnes MS, Meneghini LF, et al. Simvastatin/ Thiazolidinedione Study Group. Effects of simvastatin on the lipid profile and attainment of low-density lipoprotein cholesterol goals when added to thiazolidinedione therapy in patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2004;26:379–89. doi: 10.1016/s0149-2918(04)90033-1. [DOI] [PubMed] [Google Scholar]
- 156.Tan SA, Tan LG. Synergistic effects of simvastatin and pioglitazone on inflammatory cytokines interferon-gamma, tumor necrosis factor-alpha, interleukin-6 and C-reactive protein in diabetic patients with hyperlipidemia and coronary artery disease. Endocr Pract. 2004;10(Suppl 1):21. (Abst) [Google Scholar]
- 157.Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A PROactive Study Group. The prospective pioglitazone clinical trial in macrovascular events (PROactive): Can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes Care. 2004;27:1647–53. doi: 10.2337/diacare.27.7.1647. [DOI] [PubMed] [Google Scholar]
- 158.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999;99:178–82. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]
- 159.Conri C, Constans J, Parrot F, Skopinski S, Cipriano C. [Homocysteinemia: Role in vascular disease] Presse Med. 2000;29:737–41. [PubMed] [Google Scholar]
- 160.Seneratne MP, Griffiths J, Nagendran J. Elevation of plasma homocysteine levels associated with acute myocardial infarction. Clin Invest Med. 2000;23:220–6. [PubMed] [Google Scholar]
- 161.Bays HR, Stein EA. Pharmacotherapy for dyslipidaemia – current therapies and future agents. Expert Opin Pharmacother. 2003;4:1901–38. doi: 10.1517/14656566.4.11.1901. [DOI] [PubMed] [Google Scholar]