Abstract
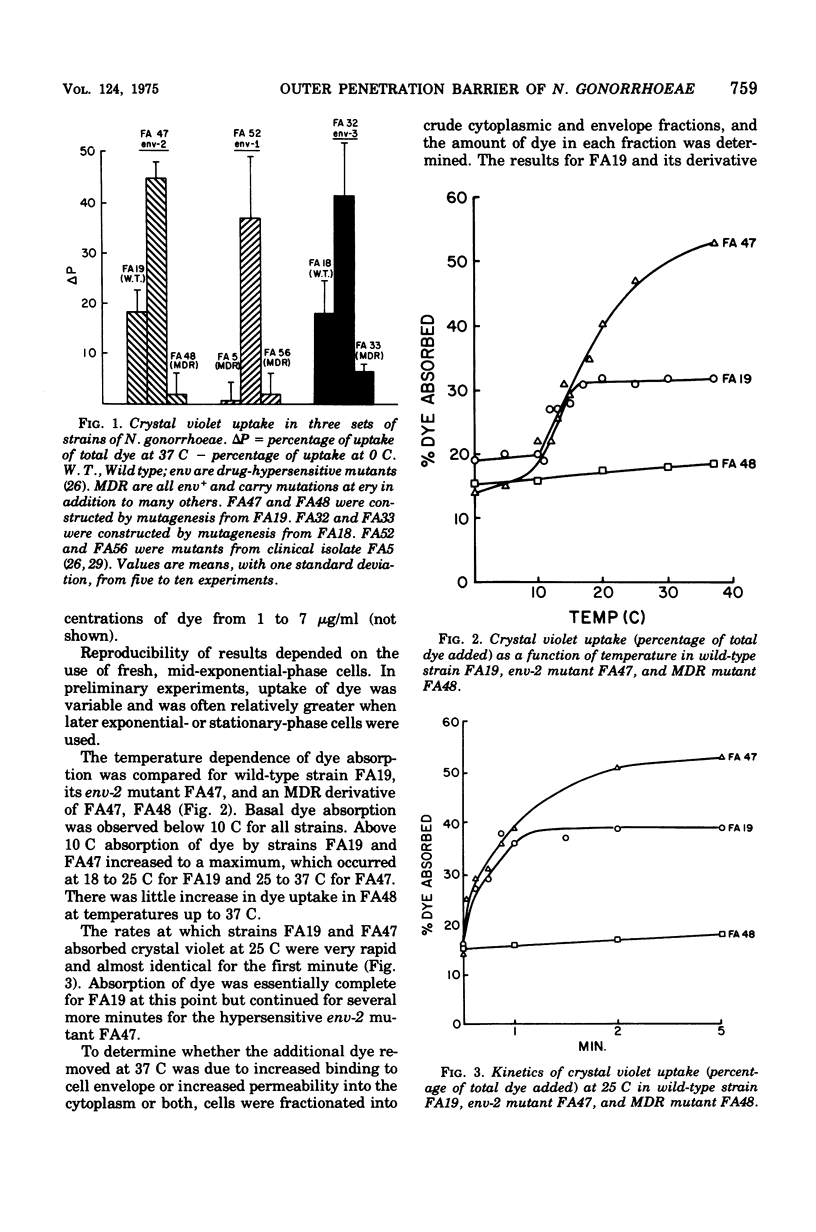
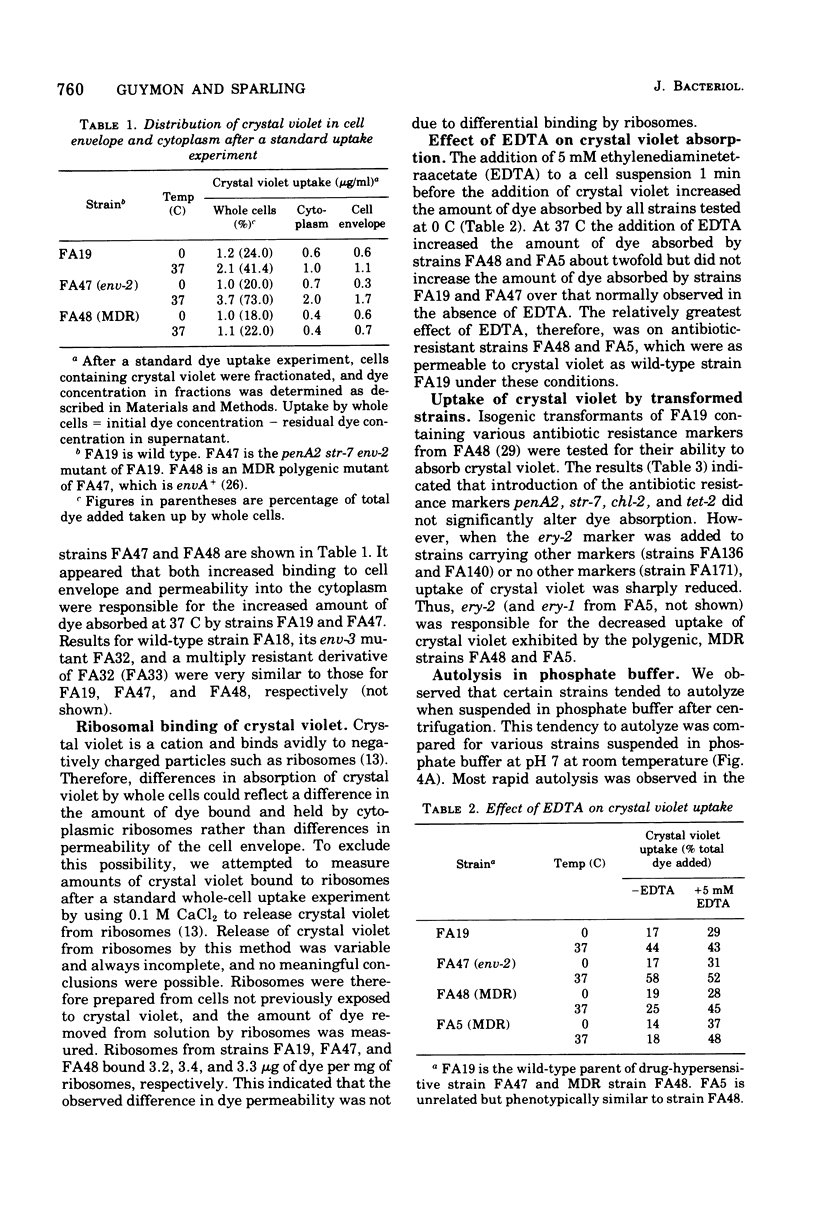
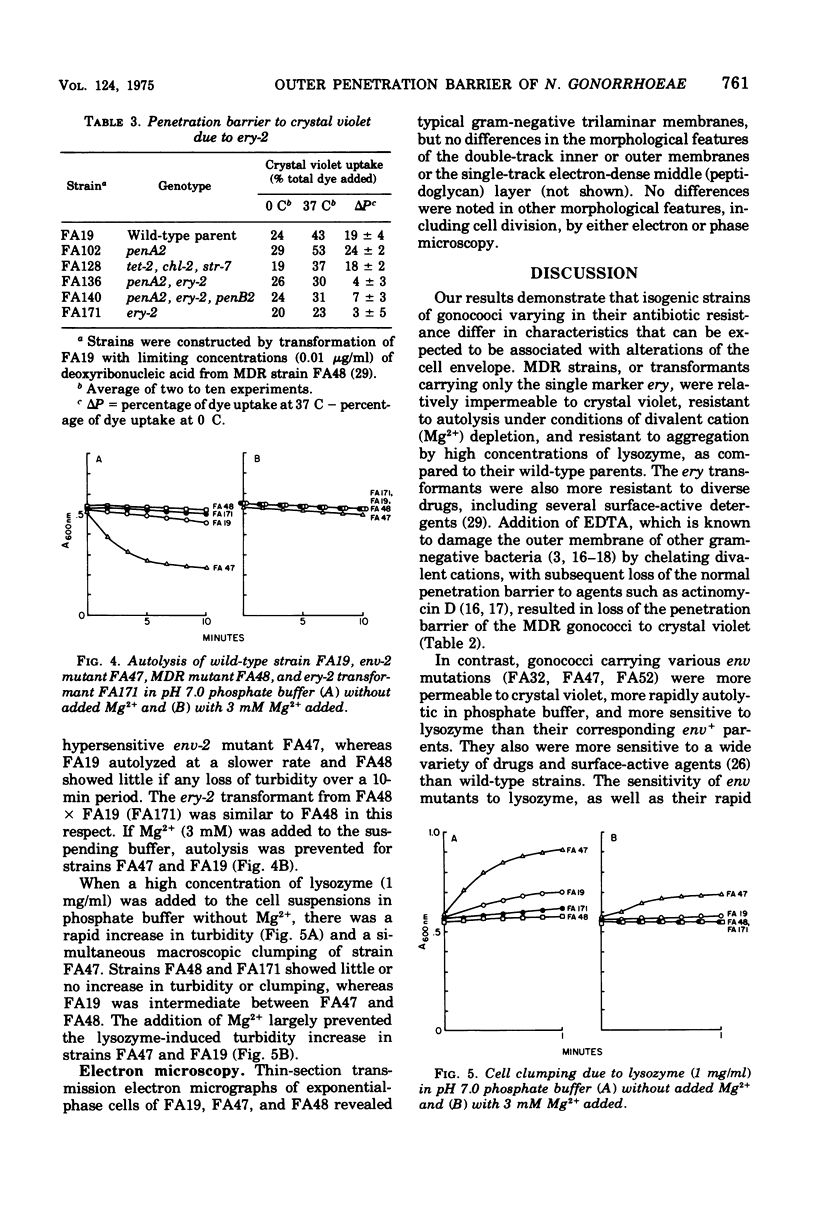
Wild-type, antibiotic-resistant and hypersensitive isogenic strains of Neisseria gonorrhoeae were studied for uptake of crystal violet, rates of autolysis, and response to lysozyme. Total uptake of crystal violet was similar in all strains at 0 C but varied significantly at 37 C. Mutation at the nonspecific resistance locus ery resulted in relative impermeability to crystal violet at 37 C, as compared to wild type. The penetration barrier to crystal violet at 37 C was overcome by addition of 5 mM ethylenediaminetetraacetic acid. Mutation at ery also resulted in reduced rates of autolysis and reduced sensitivity to high concentrations of lysozyme under conditions of divalent cation (Mg2+) depletion. In contrast, mutation at the nonspecific drug hypersensitivity locus env resulted in increased uptake of crystal violet at 37 C, due to increased binding of dye to crude envelope as well as increased penetration into cytoplasm. The env mutants were also more rapidly autolytic and more sensitive to lysozyme than wild type in the absence of Mg2+. These results suggest that the cell envelopes of ery mutants are more stable and less permeable and those of env mutants are less stable and more permeable than wild-type strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexanian S., Schlecht S., Westphal O. Untersuchungen zur Wirkung von Kristallviolett auf Salmonella-R-Mutanten. 1. Bindungsversuche. Zentralbl Bakteriol Orig A. 1974 Feb;226(2):207–214. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. The role of multivalent cations in the organization and structure of bacterial cell walls. Biochem Biophys Res Commun. 1966 Mar 22;22(6):664–671. doi: 10.1016/0006-291x(66)90198-7. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Bloom G. D. Murein and the outer penetration barrier of Escherichia coli K-12, Proteus mirabilis, and Pseudomonas aeruginosa. J Bacteriol. 1972 Dec;112(3):1364–1374. doi: 10.1128/jb.112.3.1364-1374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Young F. E. Regulation of the bacterial cell wall: isolation and characterization of peptidoglycan mutants of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):220–230. doi: 10.1128/jb.111.1.220-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin L. M., Rothman S. W., Kim R., Talevi L. A. Mechanisms and genetics of resistance to sodium lauryl sulfate in strains of Shigella and Escherichia coli. Infect Immun. 1971 Sep;4(3):287–294. doi: 10.1128/iai.4.3.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Osborn M. J. Lipopolysaccharide and aldoheptose biosynthesis in transketolase mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1673–1677. doi: 10.1073/pnas.68.8.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L., Bloomstein M. I. Discussion paper: antibiotic-sensitive mutants of Escherichia coli possess altered outer membranes. Ann N Y Acad Sci. 1974 May 10;235(0):593–600. doi: 10.1111/j.1749-6632.1974.tb43293.x. [DOI] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rogers H. J. Peptidoglycans (mucopeptides): structure, function, and variations. Ann N Y Acad Sci. 1974 May 10;235(0):29–51. doi: 10.1111/j.1749-6632.1974.tb43255.x. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Antibiotica-Empfindlichkeit bei S- und R-Formen von Salmonella minnesota. Naturwissenschaften. 1968 Oct;55(10):494–495. doi: 10.1007/BF00599721. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]
- Wu H. C. Isolation and characterization of an Escherichia coli mutant with alteration in the outer membrane porteins of the cell envelope. Biochim Biophys Acta. 1972 Dec 1;290(1):274–289. doi: 10.1016/0005-2736(72)90070-3. [DOI] [PubMed] [Google Scholar]


