Abstract
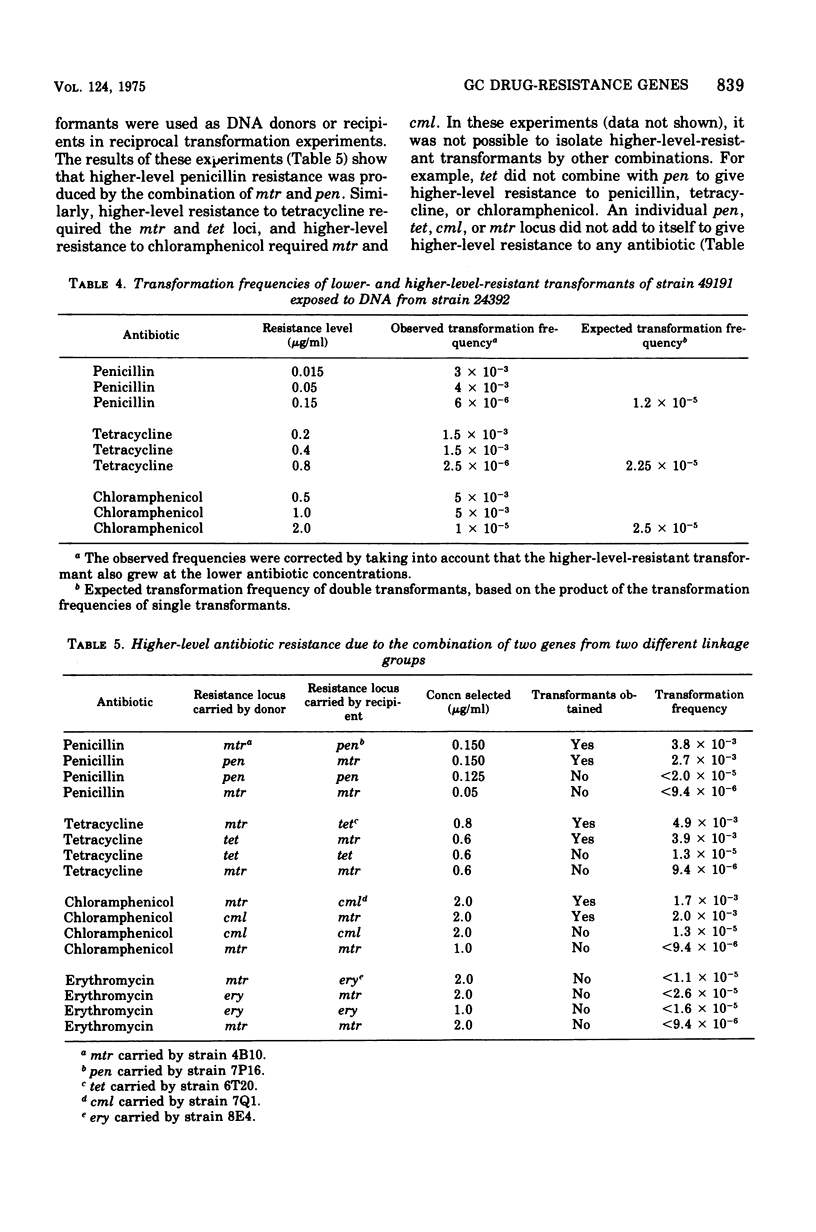
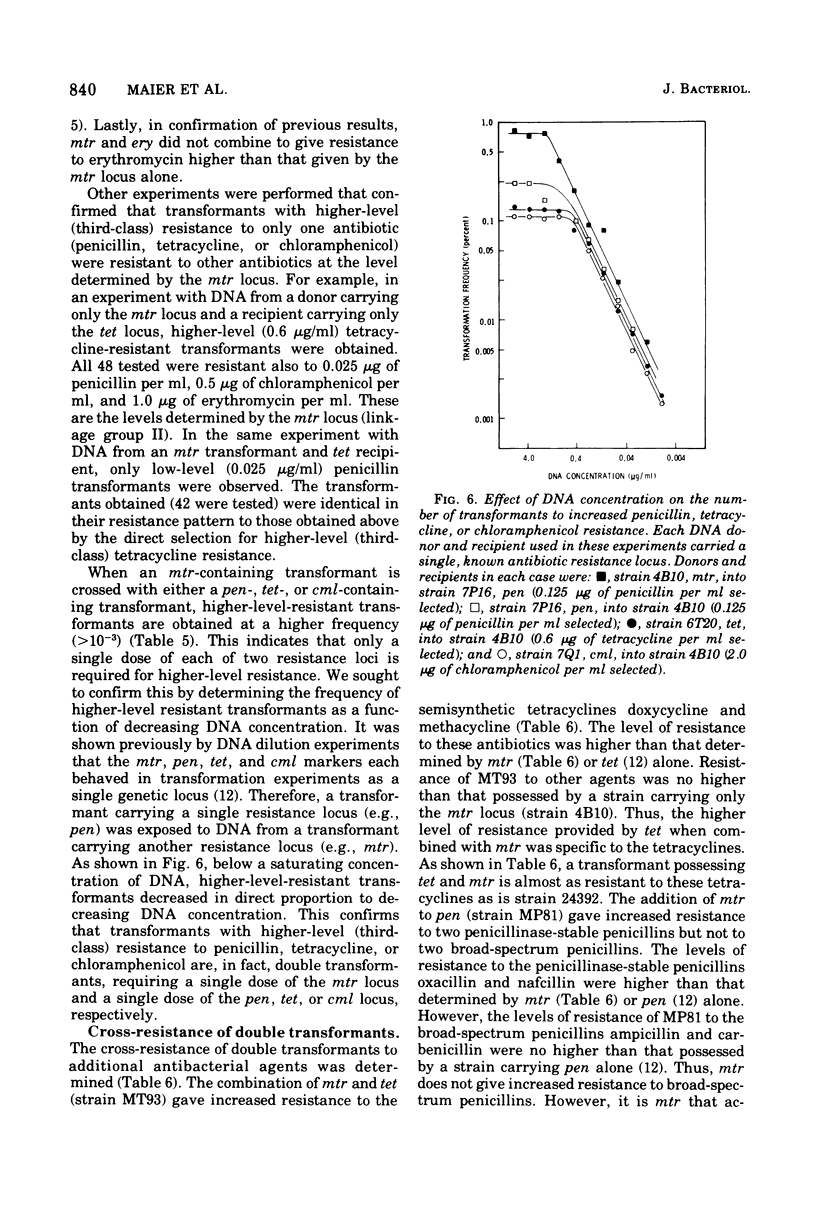
The studies reported here demonstrate that increased resistance of Neisseria gonorrhoeae to penicillin, tetracycline, and chloramphenicol results from the combined effect of two resistance loci. As shown by experiments with deoxyribonucleic acid from transformants carrying only a single resistance locus, transformants with an incresed level of resistance to penicillin result from the combination of a penicillin-specific locus, pen, and a multiple resistance locus, mtr. Similarly, transformants with an increased level of resistance to tetracycline result from the combination of mtr and a tetracycline-specific locus, tet. Transformants with an increased level of resistance to chloramphenicol result from the combination of mtr and a chloramphenicol-specific locus, cml. Deoxyribonucleic acid dilution experiments established that only a single dose of each of the two required resistance loci is necessary to give higher-level resistance. Higher-level-resistant transformants were not obtained when a double dose of one resistance locus or a combination of loci pairs other than mtr and pen, mtr and tet, or mtr and cml was introduced into a recipient. Combinations of the mtr and tet genes resulted in increased resistance to semisynthetic tetracyclines. The presence of the mtr and pen genes resulted in increased resistance to penicillinase-stable penicillins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYSON V., DEMEREC M. Patterns of resistance to antimicrobial agents. Ann N Y Acad Sci. 1950 Sep;53(2):283–289. doi: 10.1111/j.1749-6632.1950.tb42160.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Jonsson S., Monner D., Normark S., Bloom G. D. Cell-surface alterations in Escherichia coli K-12 with chromosmal mutations changing ampicillin resistance. Ann N Y Acad Sci. 1971 Jun 11;182:342–357. doi: 10.1111/j.1749-6632.1971.tb30670.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Butler L. O., Smiley M. B. Characterization by transformation of an ampicillin-resistant mutant of pneumococcus. J Gen Microbiol. 1970 May;61(2):189–195. doi: 10.1099/00221287-61-2-189. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M. Production of Staphylococcus Strains Resistant to Various Concentrations of Penicillin. Proc Natl Acad Sci U S A. 1945 Jan;31(1):16–24. doi: 10.1073/pnas.31.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M. I. Prevalence of extrachromosomal drug resistance. Changes in asusceptibility of selected pathogenic bacteria to widely used antibiotics. Ann N Y Acad Sci. 1971 Jun 11;182:5–20. doi: 10.1111/j.1749-6632.1971.tb30639.x. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Analysis of the complex sulfonamide resistance locus of pneumococcus. Cold Spring Harb Symp Quant Biol. 1958;23:85–97. doi: 10.1101/sqb.1958.023.01.012. [DOI] [PubMed] [Google Scholar]
- Maier T. W., Beilstein H. R., Zubrzycki L. Multiple antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1974 Jul;6(1):22–28. doi: 10.1128/aac.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. W., Zubrzycki L., Coyle M. B. Genetic analysis of drug resistance in Neisseria gonorrhoeae: identification and linkage relationships of loci controlling drug resistance. Antimicrob Agents Chemother. 1975 May;7(5):676–681. doi: 10.1128/aac.7.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Moses J. M., Desai M. S., Bhosle C. B., Trasi M. S. Present pattern of antibiotic sensitivity of gonococcal strains isolated in Bombay. Br J Vener Dis. 1971 Aug;47(4):273–278. doi: 10.1136/sti.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Number of transformable units per cell in Diplococcus pneumoniae. J Bacteriol. 1969 Mar;97(3):1033–1035. doi: 10.1128/jb.97.3.1033-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W., IYER V. N. The genetic relationship and phenotypic expression of mutations endowing Pneumococcus with resistance to erythromycin. J Gen Microbiol. 1961 Oct;26:277–301. doi: 10.1099/00221287-26-2-277. [DOI] [PubMed] [Google Scholar]
- Ravin A. W., Rotheim M. B., Coulter D. M. Genetic and biochemical studies of suppression of ribosomal resistance to streptomycin and erythromycin in Pneumococcus. Genetics. 1969 Jan;61(1):23–40. doi: 10.1093/genetics/61.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- Reyn A., Benzon M. W. A study of the relationships between the sensitivities of Neisseria gonorrhoeae to sodium penicillin G, four semi-synthetic penicillins, spiramycin, and fusidic acid. Br J Vener Dis. 1968 Jun;44(2):140–150. doi: 10.1136/sti.44.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W. Genetic studies on microbial cross resistance to toxic agents. IV. Cross resistance of Bacillus megaterium to forty-four antimicrobial drugs. Appl Microbiol. 1954 Mar;2(2):57–63. doi: 10.1128/am.2.2.57-63.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F. Transfer of antibiotic resistance in mixed cultures of Neisseria gonorrhoeae. J Infect Dis. 1974 Dec;130(6):660–663. doi: 10.1093/infdis/130.6.660. [DOI] [PubMed] [Google Scholar]
- Shockley T. E., Hotchkiss R. D. Stepwise introduction of transformable penicillin resistance in Pneumococcus. Genetics. 1970 Mar-Apr;64(3):397–408. [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Antibiotic resistance in Neisseria gonorrhoeae. Med Clin North Am. 1972 Sep;56(5):1133–1144. doi: 10.1016/s0025-7125(16)32339-2. [DOI] [PubMed] [Google Scholar]


