Abstract
DNA barcodes can be used to identify cryptic species of skipper butterflies previously detected by classic taxonomic methods and to provide first clues to the existence of yet other cryptic species. A striking case is the common geographically and ecologically widespread neotropical skipper butterfly Perichares philetes (Lepidoptera, Hesperiidae), described in 1775, which barcoding splits into a complex of four species in Area de Conservación Guanacaste (ACG) in northwestern Costa Rica. Three of the species are new, and all four are described. Caterpillars, pupae, and foodplants offer better distinguishing characters than do adults, whose differences are mostly average, subtle, and blurred by intraspecific variation. The caterpillars of two species are generalist grass-eaters; of the other two, specialist palm-eaters, each of which feeds on different genera. But all of these cryptic species are more specialized in their diet than was the morphospecies that held them. The four ACG taxa discovered to date belong to a panneotropical complex of at least eight species. This complex likely includes still more species, whose exposure may require barcoding. Barcoding ACG hesperiid morphospecies has increased their number by nearly 10%, an unexpectedly high figure for such relatively well known insects.
Keywords: caterpillars, foodplants, genitalia, species diversity, taxonomy
Compared with most insects, skipper butterflies (Hesperiidae) are taxonomically well known. Worldwide in occurrence, they are especially rich in neotropical species. Many of these are small, drab, and nondescript, but some are large, colorful, and showy enough to have been illustrated and formally named two or more centuries ago. Since then, a specimen that superficially seemed to fit a named species has often been assigned to it. As a consequence, the geographic distribution ascribed to a named species has usually grown over time, and repeated application of the specific epithet has tended to broaden the concept of that species. One outcome is a common, widespread, somewhat variable, but readily recognizable taxon that has become too familiar to generate further taxonomic interest. Such “weed species” warrant closer examination.
A prime example is Astraptes fulgerator, a large, common, flashy skipper described in 1775 and thought by mid-20th century to be panneotropical in distribution (1). When reared in great numbers from wild-caught dicot-eating caterpillars in Area de Conservación Guanacaste (ACG) of northwestern Costa Rica, this skipper seemed much too polyphagous for a single species. Although initial morphological examination was inconclusive, detailed comparison of large samples of adults, grouped first by what they had eaten as larvae and then by sex, revealed six or seven species differing in extremely subtle traits (e.g., shades of blue and yellow, wing shape, and size) that covaried with some ecological preferences (dry vs. rain vs. cloud forest) and different color patterns of supposedly polymorphic caterpillars. Subsequent cytochrome c oxidase (COI) DNA barcoding (2) of 466 reared adults yielded clusters that covaried with those already detected, plus three new clusters, two derived from small adult samples and one mostly from wild-caught pupae [larval foodplant(s) unknown at that time], for a total of 10 cryptic species that range from parapatric to sympatric (3).
In another analysis of reared ACG skippers, four new and very similar species (limited to rain and cloud forest) in the supposedly monotypic genus Venada were characterized by moderate differences in adult facies and male and female genitalia and by major differences in the color pattern of last-instar caterpillars. Subsequent barcoding conspicuously separated these species (4).
However, barcodes seemed not to separate closely related species within each of three pairs of skippers in the genera Polyctor, Cobalus, and Neoxeniades (5), even though notable differences in ecology (one species in ACG dry forest, the other in adjacent rain forest) and in morphology (facies, genitalia) show that the species are undeniably distinct. This apparent lack of resolution came from the use of DNA barcodes too short to include diagnostic sites. Full-length barcodes (≈650 base pairs) reliably distinguished the species in each pair of skippers, with the differences involving just one to three nucleotides. Although, in some other species, such minor barcode variation is individual, this study clearly shows that setting some arbitrary level of differentiation below which individuals are considered conspecific is untenable (6).
In each of the preceding studies, barcodes were an added character that reinforced and expanded prior conclusions reached by traditional taxonomic means. But barcodes can also provide the very first clue to the existence of unsuspected species.
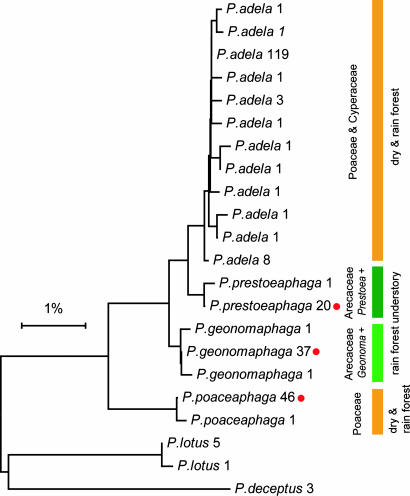
In its beginning stages, a program of systematically barcoding every ACG macrolepidopteran species (5, 7) included a few specimens of the skipper Perichares philetes. This is a large, common, distinctive, monocot-eating “weed species” that current taxonomy treats as polytypic and panneotropical. Unexpectedly, the first two P. philetes specimens to be barcoded showed a deep divergence within their conspecific cluster in a neighbor-joining (NJ) tree. This stimulated progressively more search for P. philetes caterpillars, more barcoding of P. philetes adults, and scrutiny of all life stages. Subsequent NJ trees of larger and larger samples (totaling 255 reared wild-caught individuals through mid-2007) revealed four indisputable clusters [Fig. 1 and supporting information (SI) Appendices 1 and 2].
Fig. 1.
NJ tree based on Kimura two-parameter distances for COI DNA barcodes of the six species of Perichares reared in ACG; upper four species (three new) belong to the P. philetes species complex. Numbers indicate how many individuals with each haplotype; colored bars indicate ecosystem occurrence and larval foodplants; red dots indicate the haplotypes of holotypes of the three new species.
With the circumstances reversed, the obvious taxonomic question is: What other kinds of evidence justify formal recognition of the cryptic species of Perichares indicated by DNA barcodes? A strong supportive character is larval foodplants: although all four species feed on monocots, two eat grasses, and two eat palms. The two grass-eaters use a diversity of grasses (including a few introduced species), overlap in their foodplant and microhabitat choices, and occur in both rain and dry forest; one also eats sedges. However, each of the two palm-eaters specializes on one or two species in a different genus of sympatric palms (even though many species of palms are available) and occurs only in rain forest (despite the presence of three species of indigenous palms in adjacent ACG dry forest) (see http://janzen.sas.upenn.edu and SI Appendix 1 and SI Table 2 for all specimen collateral information).
Differences in the color patterns of last-instar caterpillars and pupae further support species status (Fig. 21–12). In both stages, a pair of dorsal, longitudinal, close-set, yellow stripes runs the length of a green body. The stripes of caterpillars vary in both definition and width (stripes of grass-eaters are fuzzier and wider than are those of palm-eaters), whereas the dorsal stripes of pupae vary in their continuity and, to a lesser extent than in caterpillars, in width. Intensity of pigmentation of the caterpillar head also varies interspecifically, from very pale cream (over pale green), through medium tan, to rusty brown.
Fig. 2.
Last-instar caterpillar (in anterior and dorsal view of same individual) and pupa [in dorsal, or dorsolateral, view of different individual (except for P. prestoeaphaga)] of the six species of Perichares reared in ACG. Voucher code of each individual in parentheses. (1–3) P. adela (03-SRNP-6347, 06-SRNP-30496). (4–6) P. poaceaphaga (06-SRNP-30259, 06-SRNP-30375). (7–9) P. geonomaphaga (05-SRNP-4067, 00-SRNP-11394). (10–12) P. prestoeaphaga (00-SRNP-11749). (13) P. deceptus (03-SRNP-4930). (14–17) P. lotus [04-SRNP-11636 (including lateral view of head), 04-SRNP-13892].
Adults, on the contrary, are nearly indistinguishable in facies, even when sorted by their barcodes and sex (Fig. 3). Large sorted samples of reared specimens (with notably unworn wings) appear, en masse, to differ, but mostly in ways so subtle and variable that definitive characterization is elusive. The species differ slightly in average size, but the degree of overlap is high. Morphological identification of an isolated individual, especially if wild-caught (caterpillar foodplant unknown) and somewhat worn, is unreliable.
Fig. 3.
Males (columns one and three) and females (columns two and four) of four cryptic species of Perichares in dorsal (left) and ventral (right) view. Voucher code of each specimen in parentheses or brackets. (1–4) P. adela (06-SRNP-45166, 06-SRNP-40498). (5–8) P. poaceaphaga [06-SRNP-1189 (holotype), 03-SRNP-23215]. (9–12) P. geonomaphaga [04-SRNP-2706 (holotype), 04-SRNP-30964]. (13–16) P. prestoeaphaga [01-SRNP-22227 (holotype), 06-SRNP-31113].
Adults are sexually dimorphic with respect to the positions of yellow spots on the forewing, and males have a sizable, curved, gray, secondary sex character (Fig. 3 1, 5, 9, and 13) between the outer spots and the inner one. Many species of skippers are sexually dimorphic in wing shape, with the wings of males appreciably narrower and more pointed (and the hindwings sometimes longer) than those of females. The two palm-eating species unmistakably express this wing-shape dimorphism (one of them more perceptibly than the other), whereas the grass-eaters do not (males more closely resemble females). Exceedingly subtle sexual dimorphism relates to the quality of yellow in the forewing spots: usually, although not invariably, the yellow appears to be slightly darker in males but paler in females, except for one of the grass-eaters, in which the yellow usually appears pale in both sexes.
Within each sex, forewing spots vary considerably in size and shape, but the variation is individual. What matters taxonomically is the expression on the dorsal (although not the ventral) surface of the forewing of the lowest spot, which is relatively small and located slightly above the posterior edge of the wing. On average, this spot is better expressed in the grass- than in the palm-eaters, in one of which the spot hardly ever appears, and then only in males. On average, checkering of wing fringes is more pronounced in grass- than in palm-eaters.
Genitalia (which often distinguish related species) seem, like facies, too similar and individually variable to be dependably diagnostic for these four cryptic species. Compare minor, variable, genitalic differences between individuals of the four species with the obvious differences that characterize the other two species of Perichares in ACG (see Fig. 1 and SI Figs. 4 and 5).
One of the grass-eaters is by far the most common and polyphagous of the four similar species indicated by barcodes. It is not surprising that this grass-eater and occasional sedge-eater corresponds morphologically with what is currently classified as an extremely widespread, continental, panneotropical subspecies, P. philetes adela [ACG material matches the type of this taxon (described in 1867 from Rio de Janeiro, Brazil) in the Natural History Museum, London]. The other three species are undescribed. The specific name (a noun in apposition) given to each conveys dietary preference (e.g., the more scarce grass-eater is Perichares poaceaphaga). All four species are succinctly and comparatively described in Table 1. Although the two grass-eaters are ecologically and morphologically the most similar species, they are farthest apart in the NJ tree (compare Fig. 1 and Table 1; see also SI Appendices 1 and 2).
Table 1.
Perichares philetes species complex reared from wild-caught caterpillars in ACG, northwestern Costa Rica, including numbers of caterpillars found on each species of foodplant
| Characters | P. adela(Hewitson) | P. poaceaphaga Burns n. sp. | P. geonomaphaga Burns n. sp. | P. prestoeaphaga Burns n. sp. |
|---|---|---|---|---|
| Larval foodplants | ||||
| Cyperaceae | ||||
| Scleria latifolia | 1 | |||
| Scleria mitis | 1 | |||
| Scleria sp. | 1 | |||
| Poaceae | ||||
| Arundinella deppeana | 6 | 2 | ||
| Brachiaria sp. | 2 | |||
| Lasiacis procerrima | 1 | |||
| Lasiacis sorghoidea | 1 | |||
| Megathyrsus maximus* | 18 | 5 | ||
| Oryza latifolia | 2 | |||
| Panicum vulgaris | 1 | |||
| Paspalum botteri | 3 | |||
| Paspalum nutans | 1 | |||
| Paspalum virgatum | 62 | 3 | ||
| Paspalum sp. | 3 | |||
| Rottboellia cochinchinensis* | 5 | 1 | ||
| Saccharum spontaneum* | 2 | |||
| Setaria paniculifera | 2 | 27 | ||
| Urochloa arrecta | 4 | 1 | ||
| Undetermined spp. | 10 | 2 | ||
| Arecaceae | ||||
| Astrocaryum alatum | 2 | |||
| Chamaedorea deckeriana | 1 | |||
| Geonoma congesta | 17 | |||
| Geonoma interrupta | 49 | |||
| Prestoea decurrens | 61 | |||
| Ecosystem | Dry and rain forest | Dry and rain forest | Rain forest | Rain forest |
| Caterpillar, last instar | ||||
| Head color | Very pale tan (on pale green) (Fig. 21) | Pale tan (Fig. 24) | Rusty brown (Fig. 27) | Tan (Fig. 210) |
| Body, yellow stripes | Very wide, fuzzy (Fig. 22) | Wide, fuzzy (Fig. 25) | Narrow, quite sharp (Fig. 28) | Narrow, sharp (Fig. 211) |
| Pupa | ||||
| Body, yellow stripes | Continuous, very narrow (Fig. 23) | Continuous, narrow (Fig. 26) | Continuous, narrow (Fig. 29) | Dashed, wide (Fig. 212) |
| Adult | ||||
| Forewing length, mm | ♂ 21.46, 0.910 | ♂ 22.40, 1.120 | ♂ 23.47, 0.862 | ♂ 23.43, 1.813 |
| 19.7–23.3, 55 | 19.6–23.9, 24 | 22.2–25.0, 13 | 20.1–25.3, 9 | |
| Mean, SD | ||||
| Range, n | ♀ 23.31, 1.056 | ♀ 23.99, 0.955 | ♀ 25.54, 1.364 | ♀ 25.95, 1.895 |
| 20.2–26.0, 54 | 21.8–25.6, 19 | 22.5–27.9, 21 | 21.3–29.0, 11 | |
| Hindwing shape ♂ | Closer to ♀ (Fig. 31, 3) | Closer to ♀ (Fig. 35, 7) | Elongate, quite narrow (Fig. 39, 11) | Elongate, narrow (Fig. 313, 15) |
| Forewing yellow spots | ♂ darker ♀ lighter | ♂ and ♀ light | ♂ darker, ♀ lighter | ♂ darker, ♀ lighter |
| Forewing lowest spot, dorsal expression of | Usually sizable (Fig. 31, 2) | Sizable (Fig. 35, 6) | Usually smaller (Fig. 39, 10); absent in 5 ♀ | Absent in ♀ and in most ♂ (Fig. 313, 14); a pinpoint in 3 ♂ |
| Wing fringe checkering | Strong (Fig. 31–4) | Strong (Fig. 35–8) | Weak (Fig. 39–12) | Weak (Fig. 313–16) |
| Holotype ♂ | 06-SRNP-1189 | 04-SRNP-2706 | 01-SRNP-22227 | |
| Paratypes | 22 ♂, 17 ♀ | 11 ♂, 18 ♀ | 5 ♂, 8 ♀ |
See SI Table 2 for specimen detail.
*Introduced.
Apropos of Table 1, a cautionary note: The samples of adults are large enough to show individual variation, and it is clearly pervasive. Although reflecting real differences, the averages of wing length in the species descriptions are low, because reared adults average smaller than wild ones. Each caterpillar receives the species of plant on which it was found, but new foliage is offered only at 3- to 4-day intervals, and its quality is therefore inferior. The degree to which a reared adult is stunted depends at least in part on the instar of the caterpillar when it was collected and hence on how long it has been fed in captivity. Wild-caught caterpillars in our samples far outnumber wild-caught pupae, which presumably yield adults of natural size. Perichares follows the general rule in skippers that females are on average larger than males (for detailed documentation in unrelated hesperiid genera, see refs. 8 and 9).
Two other species of Perichares, both grass-eaters, have been reared (rarely) in ACG. Adults of these species differ conspicuously from each other and from the cryptic four (Fig. 1) (SI Appendix 2, SI Figs. 4 and 5). Although the last-instar caterpillar of Perichares deceptus has a pair of dorsal yellow stripes, they embrace a narrowly black-bordered blue central stripe (Fig. 2 13). When Perichares lotus pupates, it shows its stripes (Fig. 2 17), but the caterpillar lacks them (Fig. 2 14), and the lower side of its head has a large, elliptical, black spot (Fig. 2 16), which does not appear in the other species.
Discussion
To a great extent, current skipper classification (10) still reflects sweeping revisionary work of the mid-20th century (1), in which related species were often reduced to subspecific rank or originally described as subspecies, without adequate justification (11, 12). Thus, P. philetes became a polytypic species of four subspecies: two long-known and wide-ranging in the neotropics and two newly described and limited in distribution (essentially to southern Brazil plus Paraguay and to Peru) (1). Judging from facies, the latter are distinct species, Perichares aurina and Perichares limana, as is a supposed synonym of Perichares adela called Perichares marmorata (originally described from Venezuela). There is some sympatry among taxa. All of the above, together with the three new species from ACG, now constitute the P. philetes species complex.
P. philetes, originally described from Jamaica in 1775, is a Greater Antillean grass-eater. Like the grass-eaters in ACG, its mature caterpillar has a pair of fuzzy, wide, close-set, yellow stripes, and its pupa has “two longitudinal and parallel light yellow lines or stripes travers[ing] the dorsal surface” (13). Caterpillars have repeatedly been found feeding on sugar cane (Saccharum), an introduced grass, originally from Asia, in Cuba (14), Jamaica (13), Hispaniola (15), and Puerto Rico (16, 17). This seeming monophagy is an artifact stemming from the economic importance of the food/plant. In Jamaica, caterpillars occasionally were found on Panicum maximum and Zea (13) and, in Puerto Rico, on two bamboos, Bambusa vulgaris and Cephalostachyum pergracile (18). When hurricane Hugo devastated forests in northeastern Puerto Rico in September 1989, P. philetes caterpillars were found the next spring in large numbers on the sedge Scleria pterota, which flourished in disturbed areas; “some plants were attacked by eight or more larvae” (19). Species of Saccharum, Panicum, and Scleria are among the many foodplants of Perichares adela in ACG (Table 1).
In central Brazil, “the larva [of P. philetes], though sometimes found on [a single species in each of two genera (Desmoncus and Hyospathe) of palms] appears to favour the common sugar cane in the neighbourhood of Pará” (20). There are additional records of P. philetes on both palm and grass in Trinidad and Guyana and, again, on sugar cane in Trinidad, Venezuela, Guyana, and Argentina (18). At the very least, two species are involved. The name P. philetes does not refer to the palm-eaters, and whether it can be applied to the grass-eaters is questionable. P. philetes was reported from northwestern Argentina (21), and subspecies P. philetes philetes, from the Greater Antilles, on the one hand, and Trinidad, Tobago, the Guianas, central Brazil (Pernambuco, Pará, Amazonas), and Iquitos, Peru, on the other (1). But the southern continental populations are widely separated from the northern insular populations and may well be specifically distinct.
Grass-eaters of the P. philetes complex appear to be opportunistic generalists, whereas palm-eaters are narrow specialists. However, these specialists, unlike those in the A. fulgerator complex (3), use a single plant family (Arecaceae) instead of a diversity of unrelated plant families. That many tropical species thought to be dietary generalists actually include or comprise sets of dietary specialists is becoming increasingly evident, in part because of barcoding efforts (e.g., ref. 22). In the course of barcoding 422 morphospecies of ACG skippers, ≈40 (≈9.5%) seem to be two or more biological species. P. philetes is a spectacular case, second only to that of A. fulgerator (3).
Revelation and analysis of the P. philetes species complex are incomplete, because barcodes have been obtained inside but not outside of ACG. The massive and intensive endeavor of the ACG bioinventory cannot do both. Nevertheless, a major effort is made to sample more ACG specimens whenever incongruities appear in a NJ tree. The four species of the P. philetes complex in ACG are so irrefutably real that they must occur beyond this small area of 125,000 terrestrial ha, and some of their characters (including barcodes) may vary geographically. For now, attempts to place these species in a broader taxonomic and geographic context can only rely on fragmentary nonmolecular evidence. In its entirety, the complex probably includes additional cryptic species. All things considered, the bioinventory of ACG is opening a can of neotropical worms, or caterpillars, and catalyzing taxonomic metamorphoses.
Materials and Methods
For specifics relating to specimen collection and molecular analyses, see ref. 5.
Specimen images, collection details, and sequence records are also available in the project file “Perichares of the ACG” on the Barcode of Life Data System web site (www.barcodinglife.org) (see ref. 5). All barcoded specimens (including holotypes) have been deposited in the National Museum of Natural History, Smithsonian Institution, and may be recovered by their individual voucher codes (SI Table 2). Barcodes of holotypes and of a representative specimen of P. adela are given in SI Table 3).
Supplementary Material
Acknowledgments.
We thank Sarah Burns for helping in many and various ways; Young Sohn for drawing genitalia figures; Karie Darrow for arranging them in plates and fitting Table 1 on a single page; Lee-Ann Hayek for calculating statistics; Donald Harvey for dissecting genitalia and locating literature; Campbell Smith for photographing holotypes in the Natural History Museum, London; Tanya Dapkey for delegging and documenting specimens; Rebecca Cowling for assisting with molecular analysis; and ACG parataxonomists for finding and rearing the caterpillars. This work was supported by the National Museum of Natural History Small Grants Program (J.M.B.); by U.S. National Science Foundation Grants BSR 9024770 and DEB 9306296, 9400829, 9705072, 0072730, and 0515699; by grants from the Guanacaste Dry Forest Conservation Fund and ACG (D.H.J.); and by the Gordon and Betty Moore Foundation, the Natural Sciences and Engineering Research Council of Canada, Genome Canada through the Ontario Genomics Institute, and the Canada Research Chairs program (P.D.N.H.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (for accession nos., see SI Table 2).
This article contains supporting information online at www.pnas.org/cgi/content/full/0712181105/DC1.
References
- 1.Evans WH. London: British Museum; 1955. A Catalogue of the American Hesperiidae Indicating the Classification and Nomenclature Adopted in the British Museum (Natural History), Part IV, Hesperiinae and Megathyminae; pp. 1–499. pls 54–88. [Google Scholar]
- 2.Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc London Ser B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns JM, Janzen DH. Pan-neotropical genus Venada (Hesperiidae: Pyrginae) is not monotypic: four new species occur on one volcano in the Area de Conservación Guanacaste, Costa Rica. J Lepid Soc. 2005;59:19–34. [Google Scholar]
- 5.Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PDN. DNA barcodes distinguish species of tropical Lepidoptera. Proc Natl Acad Sci USA. 2006;103:968–971. doi: 10.1073/pnas.0510466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns JM, Janzen DH, Hajibabaei M, Hallwachs W, Hebert PDN. DNA barcodes of closely related (but morphologically and ecologically distinct) species of skipper butterflies (Hesperiidae) can differ by only one to three nucleotides. J Lepid Soc. 2007;61:138–153. [Google Scholar]
- 7.Janzen DH, Hajibabaei M, Burns JM, Hallwachs W, Remigio E, Hebert PDN. Wedding biodiversity inventory of a large and complex Lepidoptera fauna with DNA barcoding. Philos Trans R Soc London Ser B. 2005;360:1835–1845. doi: 10.1098/rstb.2005.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns JM. Evolution in skipper butterflies of the genus Erynnis. Univ Calif Publ Entomol. 1964;37:1–216. [Google Scholar]
- 9.Burns JM. Wallengrenia otho and W. egeremet in eastern North America (Lepidoptera: Hesperiidae: Hesperiinae) Smithsonian Contrib Zool. 1985;no. 423:1–39. [Google Scholar]
- 10.Mielke OHH. Hesperioidea. In: Lamas G, Heppner JB, editors. Checklist: Part 4A, Hesperioidea-Papilionoidea. 2004. pp. 3–11.pp. 25–86. Atlas Neotrop Lepid 5A. [Google Scholar]
- 11.Burns JM, Kendall RO. Ecologic and spatial distribution of Pyrgus oileus and Pyrgus philetas (Lepidoptera: Hesperiidae) at their northern distributional limits. Psyche. 1969;76:41–53. [Google Scholar]
- 12.Burns JM, Janzen DH. Biodiversity of pyrrhopygine skipper butterflies (Hesperiidae) in the Area de Conservación Guanacaste, Costa Rica. J Lepid Soc. 2001;55:15–43. [Google Scholar]
- 13.Panton ES. The life-history of some Jamaica Hesperiidae. J Inst Jamaica. 1898;2:435–441. 1 pl. [Google Scholar]
- 14.Dethier VG. Hesperiidae affecting sugar cane in Cuba. Mem Soc Cubana Hist Nat. 1942;16:167–176. pl 26. [Google Scholar]
- 15.Wolcott GN. The insects of sugar cane in Santo Domingo. J Dept Agric Labor Porto Rico. 1922;6:32–37. [Google Scholar]
- 16.Jones TH, Wolcott GN. The caterpillars which eat the leaves of sugar cane in Porto Rico. J Dept Agric Labor Porto Rico. 1922;6:38–50. [Google Scholar]
- 17.Wolcott GN. The insects of Puerto Rico. J Agric Univ Puerto Rico. 1951;32:417–748. [Google Scholar]
- 18.Cock MJW. The skipper butterflies (Hesperiidae) of Trinidad .Part 13, Hesperiinae, genera group K. Living World. J Trinidad Tobago Field Nat Club. 2005;2005:23–47. [Google Scholar]
- 19.Torres JA. Lepidoptera outbreaks in response to successional changes after the passage of Hurricane Hugo in Puerto Rico. J Trop Ecol. 1992;8:285–298. [Google Scholar]
- 20.Moss AM. Biological notes on some “Hesperiidae” of Para and the Amazon (Lep. Rhop.) Acta Zool Lilloana. 1949;7:27–79. pls I–V. [Google Scholar]
- 21.Hayward KJ, Descole HR. Genera et Species Animalium Argentinorum. Vol. 2. Buenos Aires: Guillermo Kraft; 1950. Insecta, Lepidoptera (Rhopalocera), familia Hesperiidarum, subfamilia Hesperiinarum; pp. 1–388. pls I-XXVI. [Google Scholar]
- 22.Smith MA, Wood DM, Janzen DH, Hallwachs W, Hebert PDN. DNA barcodes affirm that 16 species of apparently generalists tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc Natl Acad Sci USA. 2007;104:4967–4972. doi: 10.1073/pnas.0700050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.