Abstract
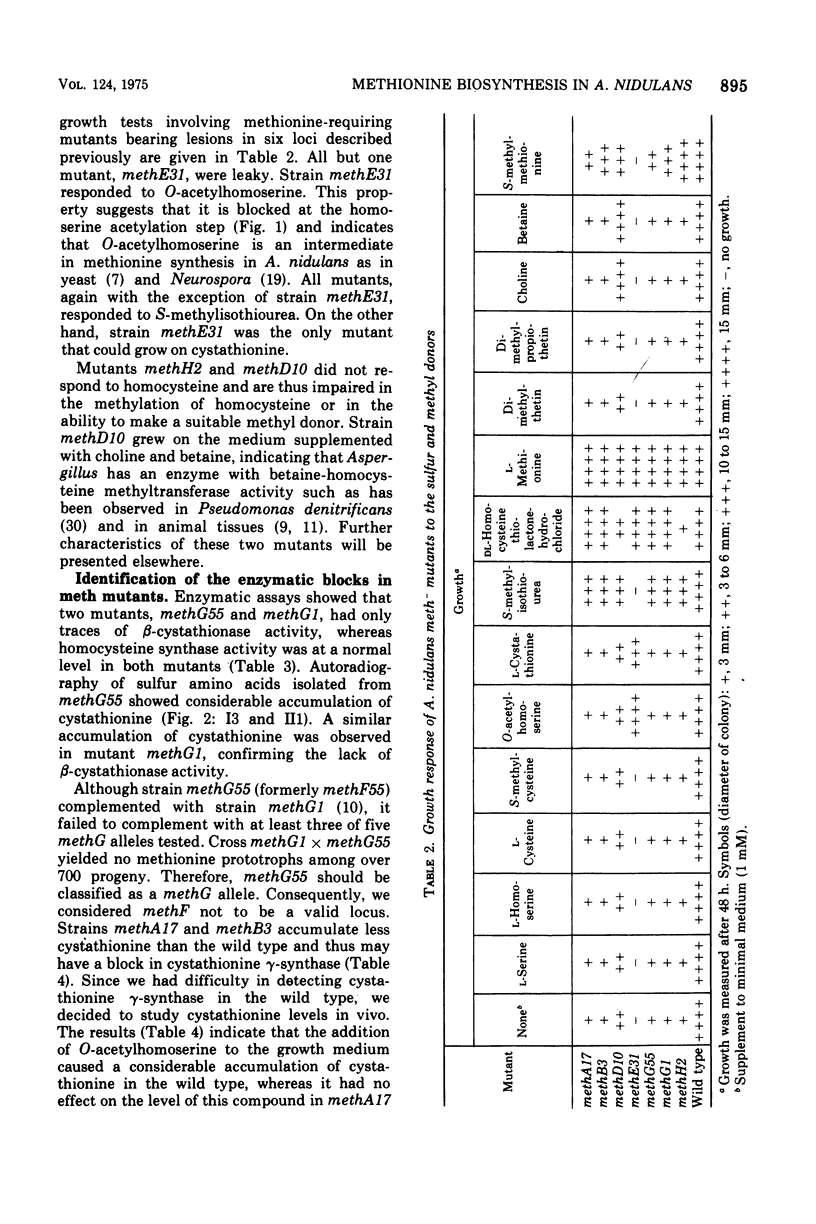
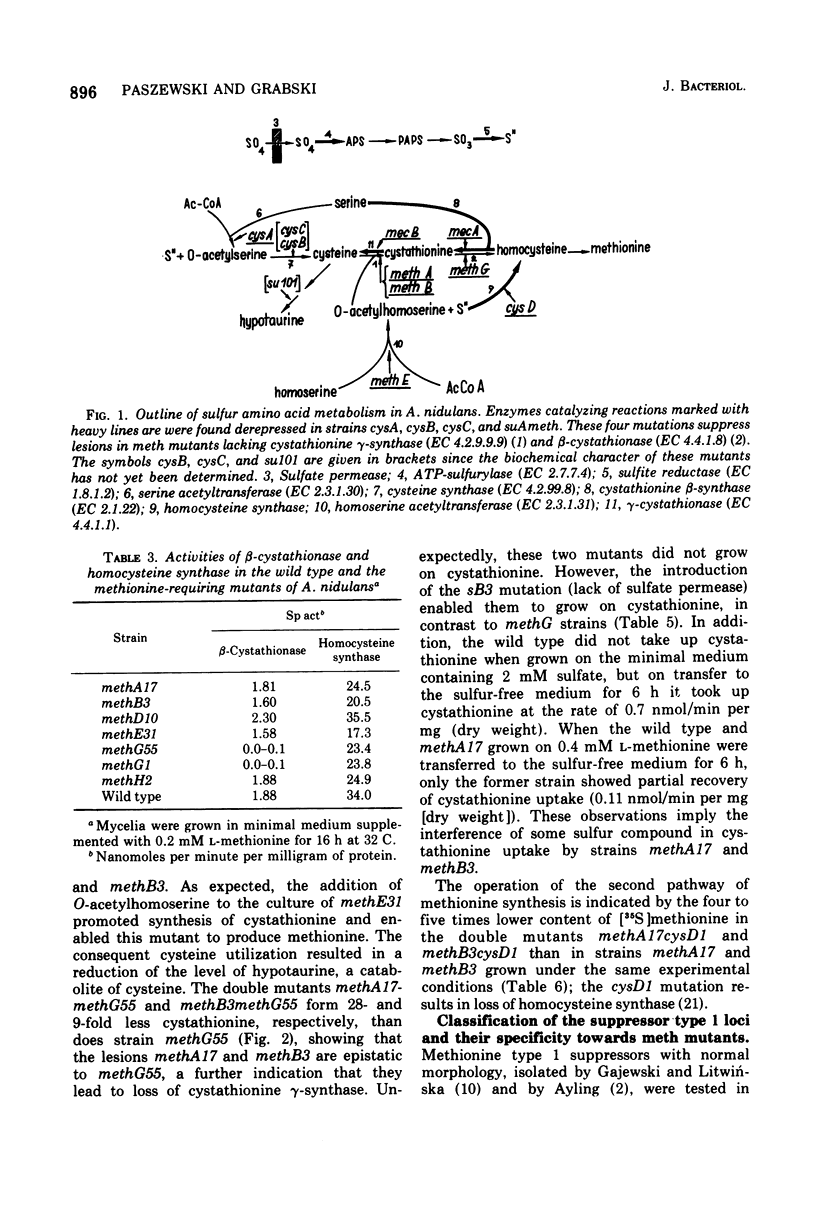
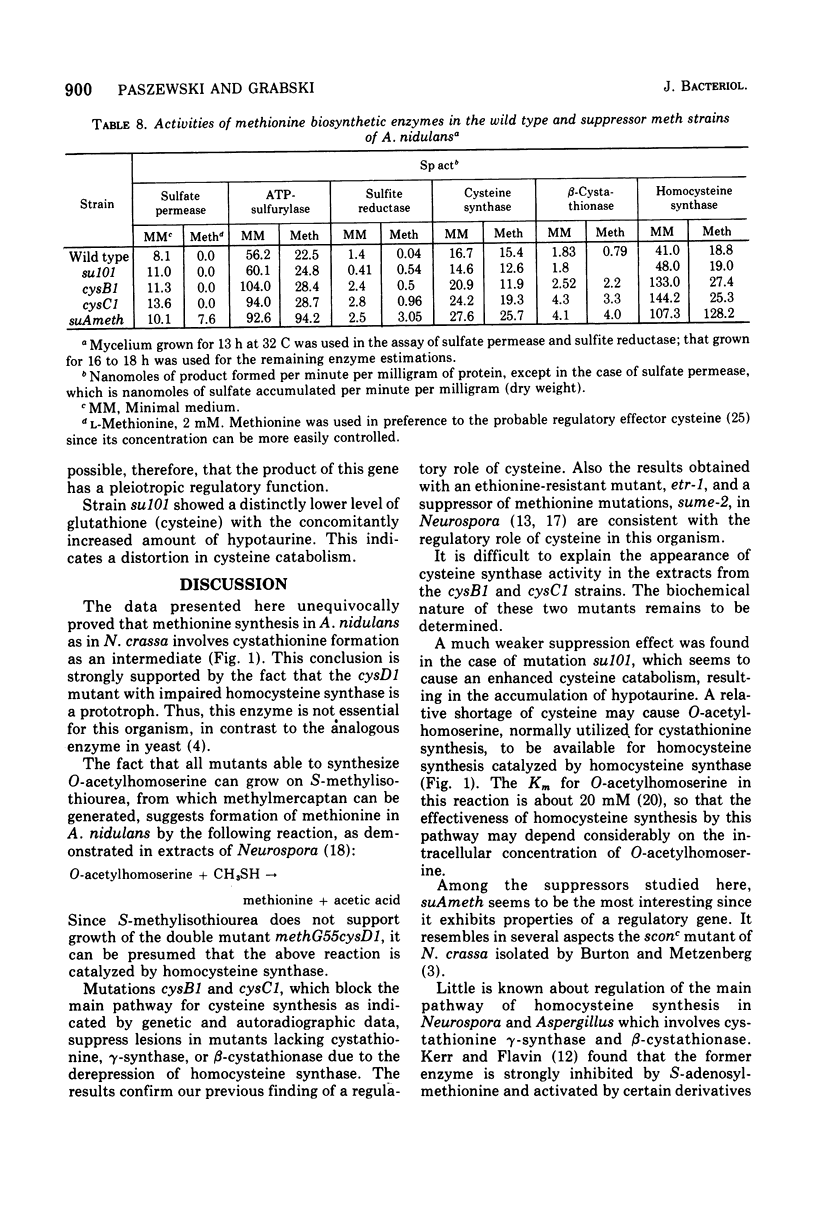
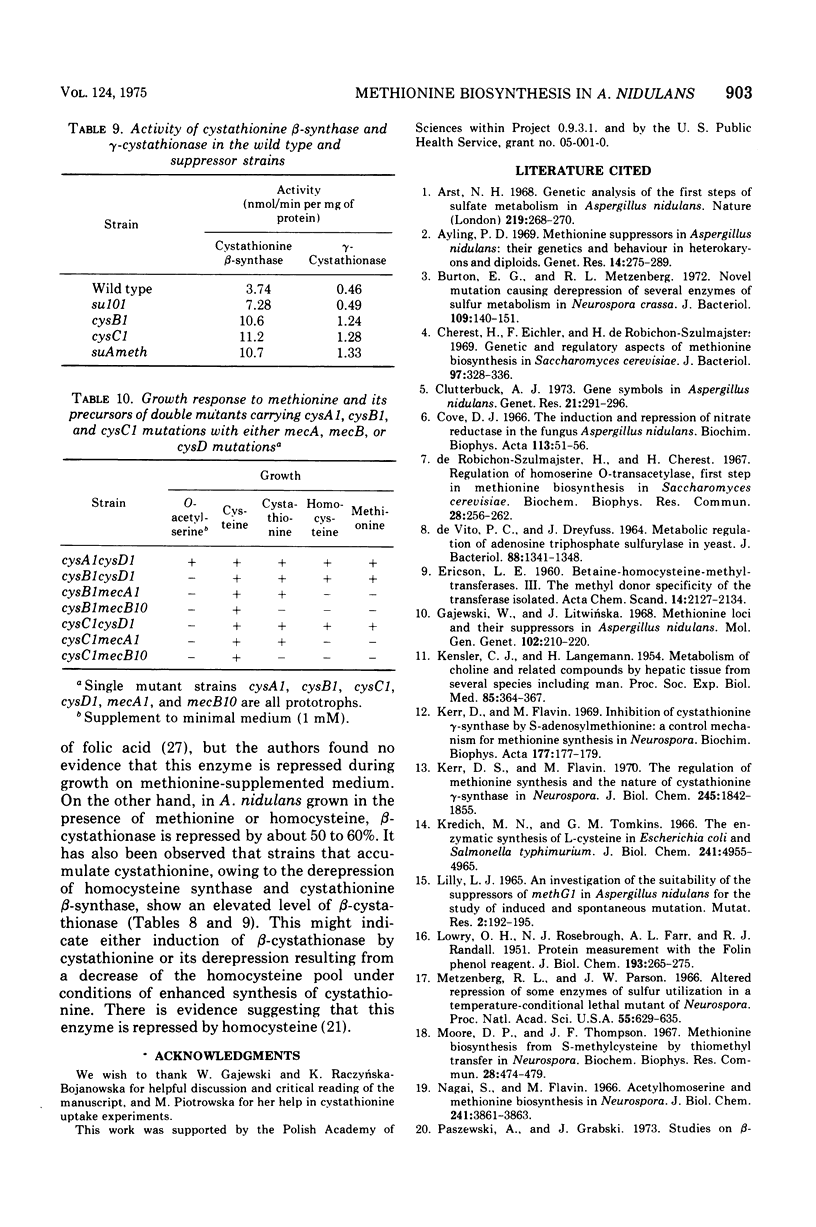
In Aspergillus nidulans the pathway involving cystathionine formation is the main one for homocysteine synthesis. Mutants lacking cystathionine gamma-synthase or beta-cystathionase are auxotrophs suppressible by: (i) mutations in the main pathway of cysteine synthesis (cysA1, cysB1, and cysC1), (ii) mutations causing stimulation of cysteine catabolism (su101), and (iii) mutations in a presumed regulatory gene (suAmeth). A relative shortage of cysteine in the first group of suppressors causes a derepression of homocysteine synthase, the enzyme involved in the alternative pathway of homocysteine synthesis. A similar derepression is observed in the suAmeth strain. Homocysteine synthesized by this pathway serves as precursor for cysteine and methionine synthesis. A mutant with altered homocysteine synthase is a prototroph, indicating that this enzyme is not essential for the fungus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature. 1968 Jul 20;219(5151):268–270. doi: 10.1038/219268a0. [DOI] [PubMed] [Google Scholar]
- Ayling P. D. Methionine suppressors in Aspergillus nidulans: their genetics and behaviour in heterokaryons and diploids. Genet Res. 1969 Dec;14(3):275–289. doi: 10.1017/s001667230000210x. [DOI] [PubMed] [Google Scholar]
- Burton E. G., Metzenberg R. L. Novel mutation causing derepression of several enzymes of sulfur metabolism in Neurospora crassa. J Bacteriol. 1972 Jan;109(1):140–151. doi: 10.1128/jb.109.1.140-151.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Gene symbols in Aspergillus nidulans. Genet Res. 1973 Jun;21(3):291–296. doi: 10.1017/s0016672300013483. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski W., Litwińska J. Methionine loci and their suppressors in Aspergillus nidulans. Mol Gen Genet. 1968;102(3):210–220. doi: 10.1007/BF00385976. [DOI] [PubMed] [Google Scholar]
- KENSLER C. J., LANGEMANN H. Metabolism of choline and related compounds by hepatic tissue from several species including man. Proc Soc Exp Biol Med. 1954 Feb;85(2):364–367. doi: 10.3181/00379727-85-20882. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- Kerr D., Flavin M. Inhibition of cystathionine gamma-synthase by S-adenosylmethionine: A control mechanism for methionine synthesis in Neurospora. Biochim Biophys Acta. 1969 Feb 18;177(1):177–179. doi: 10.1016/0304-4165(69)90086-5. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilly L. J. An investigation of the suitability of the suppressors of meth 1 in Aspergillus nidulans for the study of induced and spontaneous mutation. Mutat Res. 1965 Apr;2(2):192–195. doi: 10.1016/0027-5107(65)90047-3. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Parson J. W. Altered repression of some enzymes of sulfur utilization in a temperature-conditional lethal mutant of Neurospora. Proc Natl Acad Sci U S A. 1966 Mar;55(3):629–635. doi: 10.1073/pnas.55.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. P., Thompson J. F. Methionine biosynthesis from S-methylcysteine by thiomethyl transfer in Neurospora. Biochem Biophys Res Commun. 1967 Aug 7;28(3):474–479. doi: 10.1016/0006-291x(67)90336-1. [DOI] [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine and methionine biosynthesis in Neurospora. J Biol Chem. 1966 Aug 25;241(16):3861–3863. [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Role of cysteine and-or homocysteine as regulatory effectors. Mol Gen Genet. 1974;132(4):307–320. doi: 10.1007/BF00268571. [DOI] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Studies on beta-cystathionase and omicron-acetylhomoserine sulfhydrylase as the enzymes of alternative methionine biosynthetic pathways in Aspergillus nidulans. Acta Biochim Pol. 1973;20(2):159–168. [PubMed] [Google Scholar]
- Pieniazek N. J., Bal J., Balbin E., Stepién P. P. An Aspergillus nidulans mutant lacking serine transacetylase: evidence for two pathways of cysteine biosynthesis. Mol Gen Genet. 1974;132(4):363–366. doi: 10.1007/BF00268575. [DOI] [PubMed] [Google Scholar]
- Pieniazek N. J., Kowalska I. M., Stepień P. P. Deficiency in methionine adenosyltransferase resulting in limited repressibility of methionine biosynthetic enzymes in Aspergillus nidulans. Mol Gen Genet. 1973 Nov 22;126(4):367–374. doi: 10.1007/BF00269446. [DOI] [PubMed] [Google Scholar]
- Pieniazek N., Stepień P. P., Paszewski A. An Aspergillus nidulans mutant lacking cystathionine -synthase: identity of L-serine sulfhydrylase with cystathionine -synthase and its distinctness from O-acetyl-L-serine sulfhydrylase. Biochim Biophys Acta. 1973 Jan 24;297(1):37–47. doi: 10.1016/0304-4165(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Putrament A., Guzewska J., Pieniazek D. Further genetic characteristics of methionine mutants and their suppressors in Aspergillus nidulans. Mol Gen Genet. 1970;109(3):209–218. [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- SIEGEL L. M. A DIRECT MICRODETERMINATION FOR SULFIDE. Anal Biochem. 1965 Apr;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Selhub J., Savin M. A., Sakami W., Flavin M. Synchronization of converging metabolic pathways: activation of the Cystathionine gamma-synthase of Neurospora crassa by methyltetrahydrofolate. Proc Natl Acad Sci U S A. 1971 Feb;68(2):312–314. doi: 10.1073/pnas.68.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. F., Kaplan L., Birnbaum J. Betaine-homocysteine transmethylase in Pseudomonas denitrificans, a vitamin B 12 overproducer. J Bacteriol. 1973 Jan;113(1):218–223. doi: 10.1128/jb.113.1.218-223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]