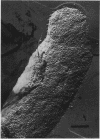
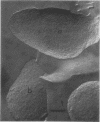
Abstract
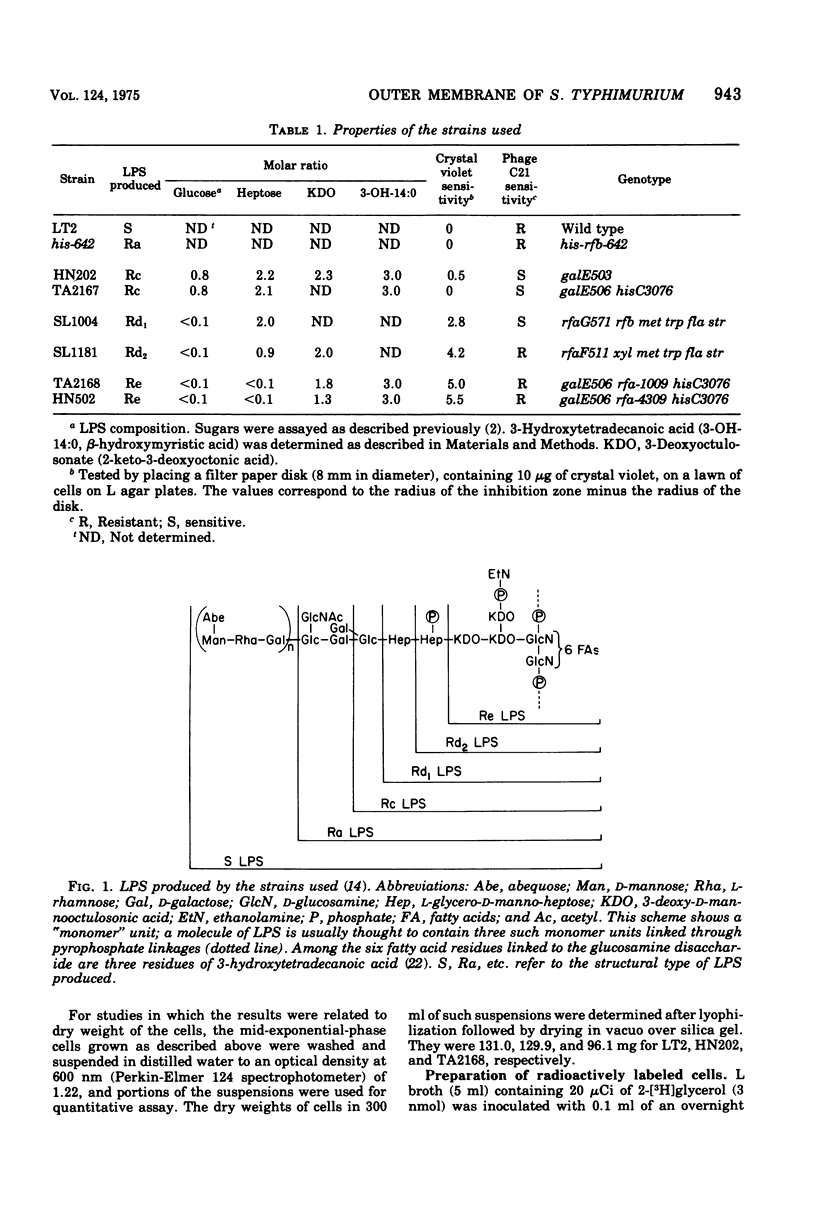
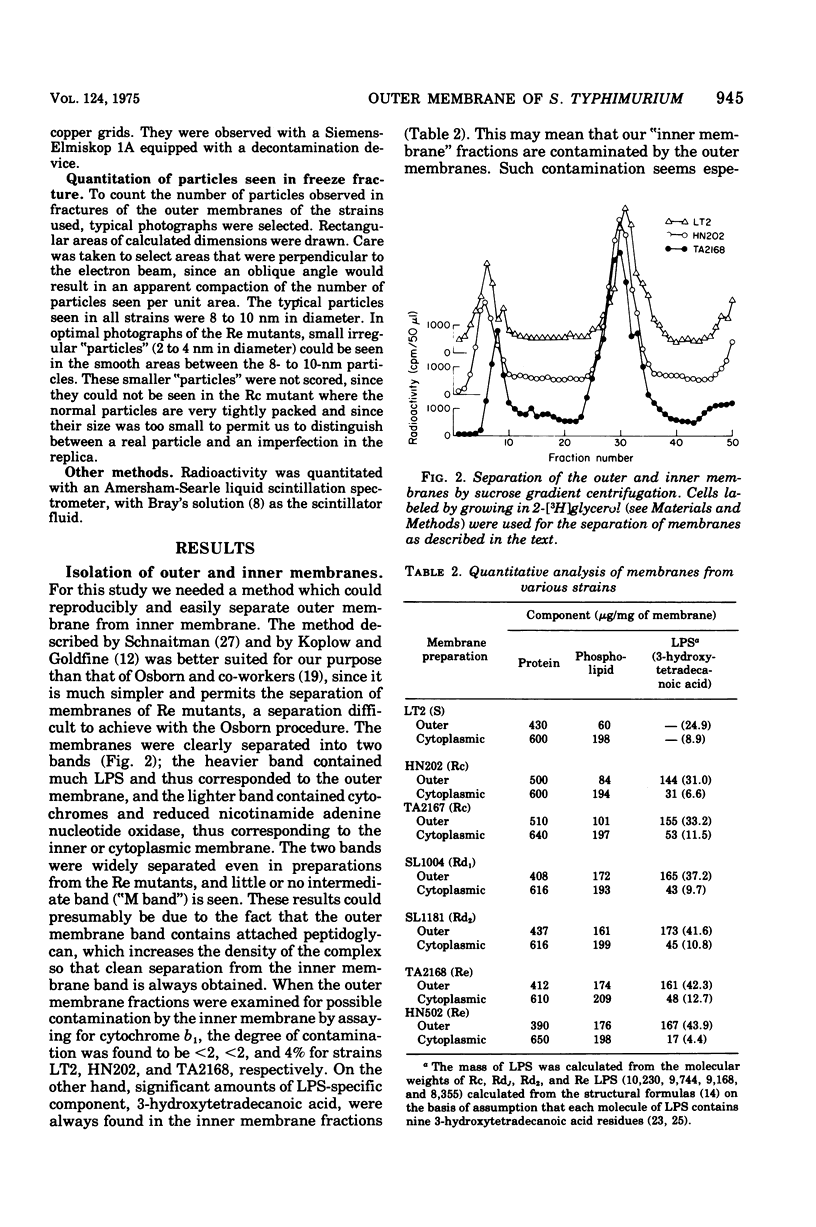
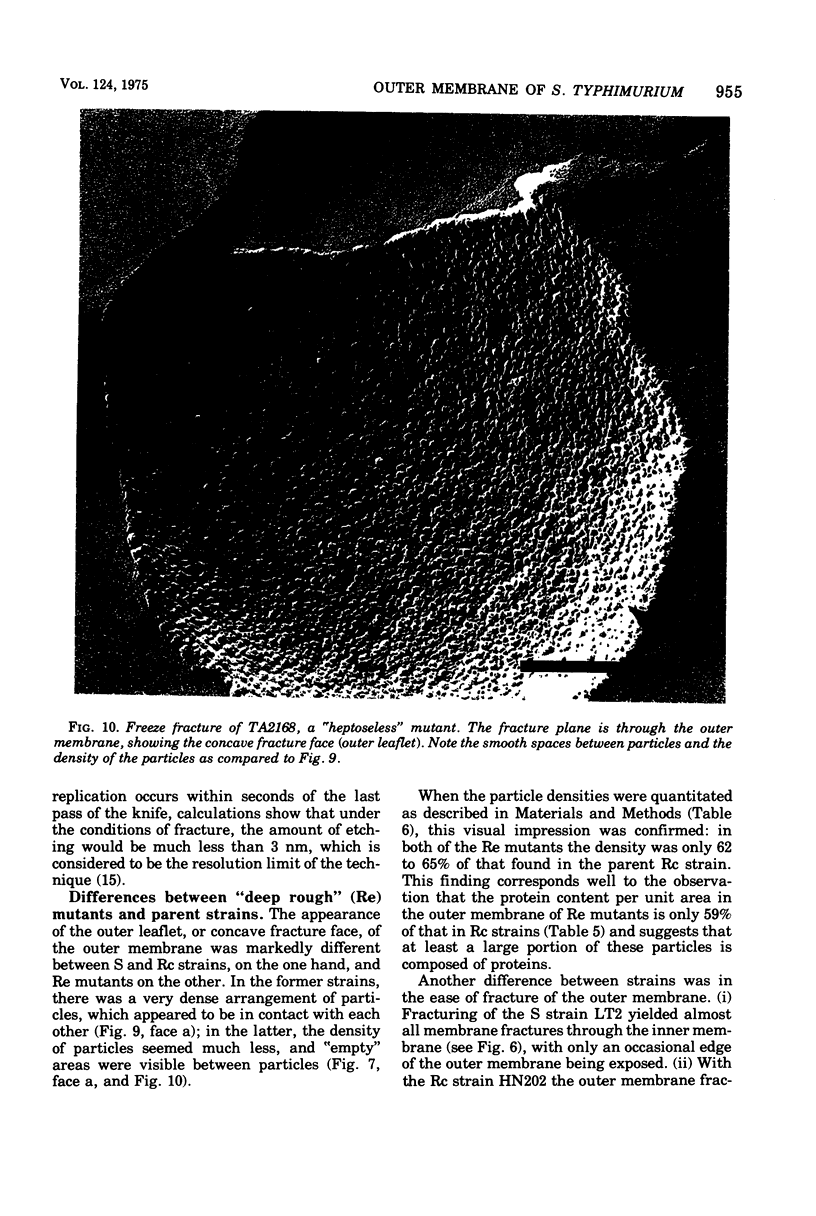
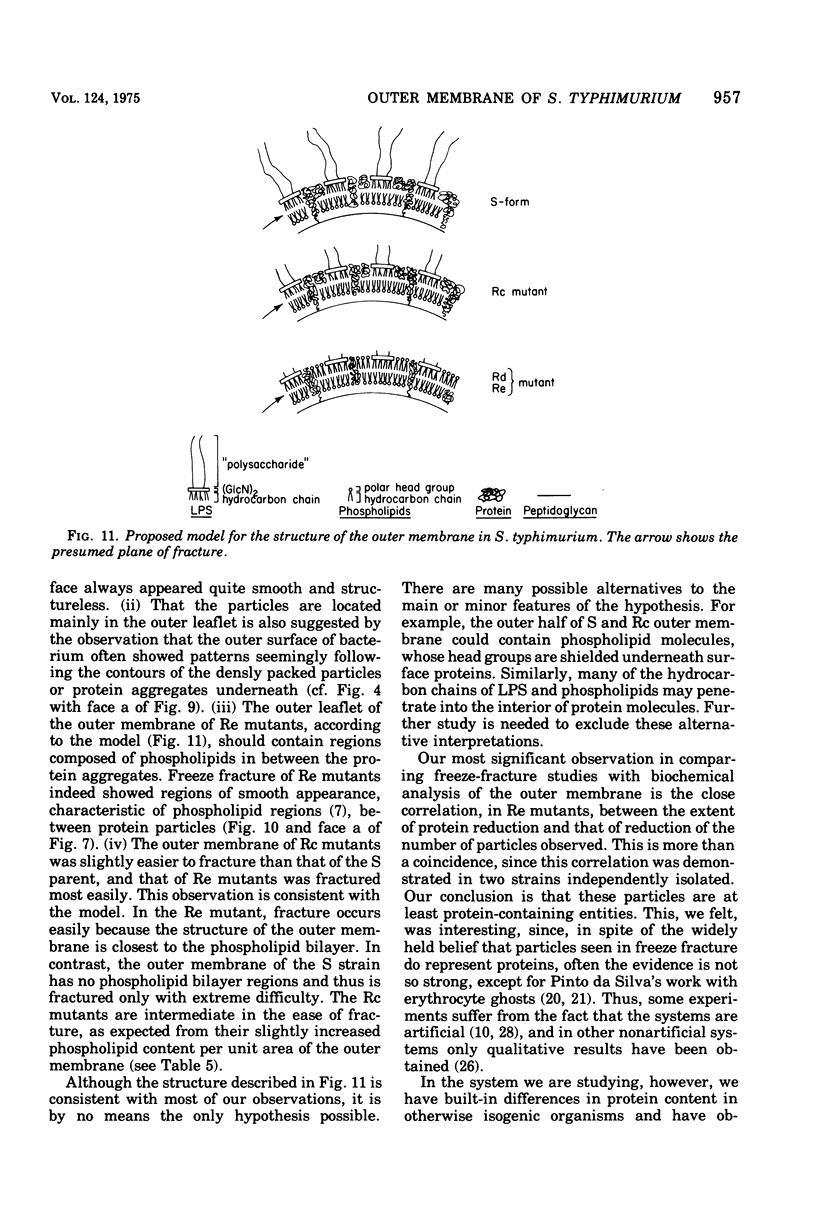
The outer membrane layer of the cell wall was isolated from wild-type Salmonella typhimurium LT2 as well as from its mutants producing lipopolysaccharides with shorter saccharide chains. Chemical analysis of these preparations indicated the following. (i) The number of lipopolysaccharide molecules per unit area was constant, regardless of the length of the saccharide side chain in lipopolysaccharide. (ii) In contrast, in "deep rough" (Rd or Re) mutants producing the lipopolysaccharides with very short saccharide chains, the amount of outer membrane protein per unit surface area decreased to about 60% of the value in the wild type. (iii) In the wild type, the amount of phospholipids is slightly less than what is needed to cover one side of the membrane as a monolayer. In comparison with the wild type, the outer membrane of Rd and Re mutants contains about 70% more phospholipids, which therefore must be distributed in both the outer and inner leaflets of the membrane. Freeze-fracture studies showed that the outer membrane of Re mutants were easily fractured, but fracture became increasingly difficult in strains producing lipopolysaccharides with longer side chains. The convex fracture face was always nearly smooth, but the concave fracture face or the outer half of the membrane was densely covered with particles 8 to 10 nm in diameter. The density of particles was decreased in Re mutants to the same extent as the reduction in proteins, suggesting the largely proteinaceous nature of particles. A model for the supramolecular structure of the outer membrane is presented on the basis of these and other results.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Preparation and properties of phospholipid bilayers containing rhodopsin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2617–2621. doi: 10.1073/pnas.69.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moor H. Freeze-etching. Int Rev Cytol. 1969;25:391–412. doi: 10.1016/s0074-7696(08)60209-0. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974 Apr 16;43(3):533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Preservation of the ultrastructure of Bacillus subtilis by chemical fixation as verified by freeze-etching. J Cell Biol. 1969 Sep;42(3):733–744. doi: 10.1083/jcb.42.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Fudenberg H. H. Anionic sites on the membrane intercalated particles of human erythrocyte ghost membranes. Freeze-etch localization. Exp Cell Res. 1973 Sep;81(1):127–138. doi: 10.1016/0014-4827(73)90119-5. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W. Freeze-etching of bacteria. Int Rev Cytol. 1972;33:253–296. doi: 10.1016/s0074-7696(08)61452-7. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Gulik-Krzywicki T., Sardet C. Association of the membrane-penetrating polypeptide segment of the human erythrocyte MN-glycoprotein with phospholipid bilayers. I. Formation of freeze-etch intramembranous particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3294–3298. doi: 10.1073/pnas.71.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]